Glucose deprivation induces chemoresistance in colorectal cancer cells by increasing ATF4 expression
- PMID: 27468213
- PMCID: PMC4945982
- DOI: 10.3748/wjg.v22.i27.6235
Glucose deprivation induces chemoresistance in colorectal cancer cells by increasing ATF4 expression
Abstract
Aim: To investigate the role of activating transcription factor 4 (ATF4) in glucose deprivation (GD) induced colorectal cancer (CRC) drug resistance and the mechanism involved.
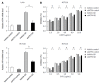
Methods: Chemosensitivity and apoptosis were measured under the GD condition. Inhibition of ATF4 using short hairpin RNA in CRC cells under the GD condition and in ATF4-overexpressing CRC cells was performed to identify the role of ATF4 in the GD induced chemoresistance. Quantitative real-time RT-PCR and Western blot were used to detect the mRNA and protein expression of drug resistance gene 1 (MDR1), respectively.
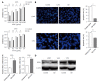
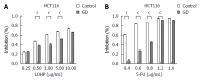
Results: GD protected CRC cells from drug-induced apoptosis (oxaliplatin and 5-fluorouracil) and induced the expression of ATF4, a key gene of the unfolded protein response. Depletion of ATF4 in CRC cells under the GD condition can induce apoptosis and drug re-sensitization. Similarly, inhibition of ATF4 in the ATF4-overexpressing CRC cells reintroduced therapeutic sensitivity and apoptosis. In addition, increased MDR1 expression was observed in GD-treated CRC cells.
Conclusion: These data indicate that GD promotes chemoresistance in CRC cells through up-regulating ATF4 expression.
Keywords: 5-Fluorouracil; ATF4; Chemoresistance; Glucose deprivation; Oxaliplatin.
Figures





Similar articles
-
Nuclear orphan receptor NR4A2 confers chemoresistance and predicts unfavorable prognosis of colorectal carcinoma patients who received postoperative chemotherapy.Eur J Cancer. 2013 Nov;49(16):3420-30. doi: 10.1016/j.ejca.2013.06.001. Epub 2013 Jun 25. Eur J Cancer. 2013. PMID: 23809767
-
ATF4-mediated microRNA-145/HDAC4/p53 axis affects resistance of colorectal cancer cells to 5-fluorouracil by regulating autophagy.Cancer Chemother Pharmacol. 2022 May;89(5):595-607. doi: 10.1007/s00280-021-04393-0. Epub 2022 Mar 21. Cancer Chemother Pharmacol. 2022. PMID: 35312836
-
Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer.BMC Cancer. 2018 Feb 20;18(1):207. doi: 10.1186/s12885-018-4123-1. BMC Cancer. 2018. PMID: 29463225 Free PMC article.
-
An Overview of Research Advances in Oncology Regarding the Transcription Factor ATF4.Curr Drug Targets. 2025;26(1):59-72. doi: 10.2174/0113894501328461240921062056. Curr Drug Targets. 2025. PMID: 39350552 Review.
-
Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells.Trends Endocrinol Metab. 2017 Nov;28(11):794-806. doi: 10.1016/j.tem.2017.07.003. Epub 2017 Aug 7. Trends Endocrinol Metab. 2017. PMID: 28797581 Free PMC article. Review.
Cited by
-
Overcoming Hypoxia-Induced Chemoresistance in Cancer Using a Novel Glycoconjugate of Methotrexate.Pharmaceuticals (Basel). 2020 Dec 24;14(1):13. doi: 10.3390/ph14010013. Pharmaceuticals (Basel). 2020. PMID: 33374474 Free PMC article.
-
Thapsigargin promotes colorectal cancer cell migration through upregulation of lncRNA MALAT1.Oncol Rep. 2020 Apr;43(4):1245-1255. doi: 10.3892/or.2020.7502. Epub 2020 Feb 13. Oncol Rep. 2020. PMID: 32323831 Free PMC article.
-
A bioinformatic analysis found low expression and clinical significance of ATF4 in breast cancer.Heliyon. 2024 Jan 12;10(2):e24669. doi: 10.1016/j.heliyon.2024.e24669. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312639 Free PMC article.
-
Overexpression of miR-1283 Inhibits Cell Proliferation and Invasion of Glioma Cells by Targeting ATF4.Oncol Res. 2019 Feb 21;27(3):325-334. doi: 10.3727/096504018X15251282086836. Epub 2018 May 1. Oncol Res. 2019. PMID: 29716673 Free PMC article.
-
New Hope for Pancreatic Ductal Adenocarcinoma Treatment Targeting Endoplasmic Reticulum Stress Response: A Systematic Review.Int J Mol Sci. 2018 Aug 21;19(9):2468. doi: 10.3390/ijms19092468. Int J Mol Sci. 2018. PMID: 30134550 Free PMC article.
References
-
- Yin Y, Zhang B, Wang W, Fei B, Quan C, Zhang J, Song M, Bian Z, Wang Q, Ni S, et al. miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 2014;20:6187–6199. - PubMed
-
- Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. - PubMed
-
- Zhu H, Chen X, Chen B, Chen B, Fan J, Song W, Xie Z, Jiang D, Li Q, Zhou M, et al. Activating transcription factor 4 mediates a multidrug resistance phenotype of esophageal squamous cell carcinoma cells through transactivation of STAT3 expression. Cancer Lett. 2014;354:142–152. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

