A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US
- PMID: 27468241
- PMCID: PMC4946862
- DOI: 10.2147/JAA.S98172
A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US
Abstract
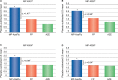
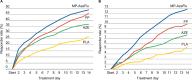
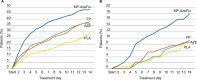
A novel intranasal formulation of azelastine HCl (AZE, an antihistamine) and fluticasone propionate (FP, a corticosteroid) in a single spray (MP-AzeFlu [Dymista®]) was studied in four randomized, double-blind, placebo-controlled trials of patients with seasonal allergic rhinitis conducted in the US. Study sites were distributed so that all major US geographic regions and the prevalent pollens within these regions were represented. Spring and summer studies included patients aged 12 years and older with allergy to grass and tree pollens. Fall studies enrolled patients with allergy to weeds, in particular ragweed. In addition, a study was conducted during the winter months in patients with allergy to mountain cedar pollen in TX, USA. Regardless of allergy season or prevalent pollen, MP-AzeFlu improved nasal symptoms of allergic rhinitis (AR) to a significantly greater degree than AZE or FP, two treatments that currently are recommended as the first-line AR therapy. MP-AzeFlu improved all individual AR symptoms and was significantly better than FP and AZE for nasal congestion relief, which is generally accepted as the most bothersome symptom for AR patients. The onset of action was within 30 minutes. MP-AzeFlu also provided clinically important improvement in the overall Rhinoconjunctivitis Quality of Life Questionnaire score and significantly improved ocular symptoms of rhinitis compared to placebo. Favorable characteristics of the MP-AzeFlu formulation as well as superior clinical efficacy make it an ideal intranasal therapy for AR.
Keywords: Dymista; Texas mountain cedar; grass pollen; intranasal therapy; ragweed; seasonal allergic rhinitis.
Figures
Similar articles
-
Efficacy of a Novel Intranasal Formulation of Azelastine Hydrochloride and Fluticasone Propionate, Delivered in a Single Spray, for the Treatment of Seasonal Allergic Rhinitis: Results from Russia.Int Arch Allergy Immunol. 2019;178(3):255-263. doi: 10.1159/000494507. Epub 2019 Jan 24. Int Arch Allergy Immunol. 2019. PMID: 30677766 Clinical Trial.
-
Multicentre, non-interventional study to assess the profile of patients with uncontrolled rhinitis prescribed a novel formulation of azelastine hydrochloride and fluticasone propionate in a single spray in routine clinical practice in the UK.BMJ Open. 2017 Apr 24;7(4):e014777. doi: 10.1136/bmjopen-2016-014777. BMJ Open. 2017. PMID: 28442578 Free PMC article.
-
Efficacy of MP-AzeFlu in children with seasonal allergic rhinitis: Importance of paediatric symptom assessment.Pediatr Allergy Immunol. 2016 Mar;27(2):126-33. doi: 10.1111/pai.12540. Pediatr Allergy Immunol. 2016. PMID: 26928753 Clinical Trial.
-
MP-AzeFlu in Moderate-to-Severe Allergic Rhinitis: A Literature Review.Int Arch Allergy Immunol. 2021;182(11):1026-1035. doi: 10.1159/000516417. Epub 2021 Jun 3. Int Arch Allergy Immunol. 2021. PMID: 34082425
-
The Use of Azelastine Hydrochloride/Fluticasone Propionate in the Management of Allergic Rhinitis in Asia: A Review.J Asthma Allergy. 2024 Jul 12;17:667-679. doi: 10.2147/JAA.S451733. eCollection 2024. J Asthma Allergy. 2024. PMID: 39045291 Free PMC article. Review.
Cited by
-
Effect of Specific Immunoglobulin E Response and Comorbidities on Effectiveness of MP-AzeFlu in a Real-Life Study.Int Arch Allergy Immunol. 2020;181(10):754-764. doi: 10.1159/000508749. Epub 2020 Aug 21. Int Arch Allergy Immunol. 2020. PMID: 32829329 Free PMC article.
-
Allergic rhinitis and asthma symptoms in a real-life study of MP-AzeFlu to treat multimorbid allergic rhinitis and asthma.Clin Mol Allergy. 2020 Aug 6;18:15. doi: 10.1186/s12948-020-00130-9. eCollection 2020. Clin Mol Allergy. 2020. PMID: 32782442 Free PMC article.
-
Superior effect of MP-AzeFlu than azelastine or fluticasone propionate alone on reducing inflammatory markers.Allergy Asthma Clin Immunol. 2018 Dec 18;14:86. doi: 10.1186/s13223-018-0311-4. eCollection 2018. Allergy Asthma Clin Immunol. 2018. PMID: 30574167 Free PMC article.
-
Turkish Guideline for Diagnosis and Treatment of Allergic Rhinitis (ART).Turk Arch Otorhinolaryngol. 2021 May;59(Suppl 1):1-157. doi: 10.4274/tao.2021.suppl.1. Turk Arch Otorhinolaryngol. 2021. PMID: 34212158 Free PMC article.
-
Pharmacokinetic Profile of Intra-articular Fluticasone Propionate Microparticles in Beagle Dog Knees.Cartilage. 2019 Apr;10(2):139-147. doi: 10.1177/1947603517723687. Epub 2017 Aug 8. Cartilage. 2019. PMID: 28786292 Free PMC article.
References
-
- Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1–S84. - PubMed
-
- Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600–608. - PubMed
-
- Dalal AA, Stanford R, Henry H, Borah B. Economic burden of rhinitis in managed care: a retrospective claims data analysis. Ann Allergy Asthma Immunol. 2008;101(1):23–29. - PubMed
-
- Levetin E, Van de Water P. Changing pollen types/concentrations/distribution in the United States: fact or fiction? Curr Allergy Asthma Rep. 2008;8(5):418–424. - PubMed
-
- Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88(3):279–282. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

