A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US
- PMID: 27468241
- PMCID: PMC4946862
- DOI: 10.2147/JAA.S98172
A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US
Abstract
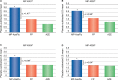
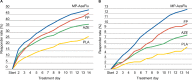
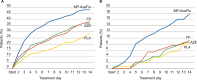
A novel intranasal formulation of azelastine HCl (AZE, an antihistamine) and fluticasone propionate (FP, a corticosteroid) in a single spray (MP-AzeFlu [Dymista®]) was studied in four randomized, double-blind, placebo-controlled trials of patients with seasonal allergic rhinitis conducted in the US. Study sites were distributed so that all major US geographic regions and the prevalent pollens within these regions were represented. Spring and summer studies included patients aged 12 years and older with allergy to grass and tree pollens. Fall studies enrolled patients with allergy to weeds, in particular ragweed. In addition, a study was conducted during the winter months in patients with allergy to mountain cedar pollen in TX, USA. Regardless of allergy season or prevalent pollen, MP-AzeFlu improved nasal symptoms of allergic rhinitis (AR) to a significantly greater degree than AZE or FP, two treatments that currently are recommended as the first-line AR therapy. MP-AzeFlu improved all individual AR symptoms and was significantly better than FP and AZE for nasal congestion relief, which is generally accepted as the most bothersome symptom for AR patients. The onset of action was within 30 minutes. MP-AzeFlu also provided clinically important improvement in the overall Rhinoconjunctivitis Quality of Life Questionnaire score and significantly improved ocular symptoms of rhinitis compared to placebo. Favorable characteristics of the MP-AzeFlu formulation as well as superior clinical efficacy make it an ideal intranasal therapy for AR.
Keywords: Dymista; Texas mountain cedar; grass pollen; intranasal therapy; ragweed; seasonal allergic rhinitis.
Figures
References
-
- Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1–S84. - PubMed
-
- Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600–608. - PubMed
-
- Dalal AA, Stanford R, Henry H, Borah B. Economic burden of rhinitis in managed care: a retrospective claims data analysis. Ann Allergy Asthma Immunol. 2008;101(1):23–29. - PubMed
-
- Levetin E, Van de Water P. Changing pollen types/concentrations/distribution in the United States: fact or fiction? Curr Allergy Asthma Rep. 2008;8(5):418–424. - PubMed
-
- Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88(3):279–282. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

