Ferumoxytol versus placebo in iron deficiency anemia: efficacy, safety, and quality of life in patients with gastrointestinal disorders
- PMID: 27468245
- PMCID: PMC4946860
- DOI: 10.2147/CEG.S101473
Ferumoxytol versus placebo in iron deficiency anemia: efficacy, safety, and quality of life in patients with gastrointestinal disorders
Abstract
Introduction: Iron deficiency anemia (IDA) is common in patients with gastrointestinal (GI) disorders and can adversely affect quality of life. Oral iron is poorly tolerated in many patients with GI disorders. Ferumoxytol is approved for the intravenous treatment of IDA in patients with chronic kidney disease. This study aimed to evaluate the efficacy and safety of ferumoxytol in patients with IDA and concomitant GI disorders.
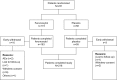
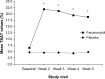
Patients and methods: This analysis included 231 patients with IDA and GI disorders from a Phase III, randomized, double-blind, placebo-controlled trial evaluating ferumoxytol (510 mg ×2) versus placebo in patients who had failed or were intolerant of oral iron therapy. The primary study end point was the proportion of patients achieving a ≥20 g/L increase in hemoglobin (Hgb) from baseline to Week 5. Other end points included mean change in Hgb, proportion of patients achieving Hgb ≥120 g/L, mean change in transferrin saturation, and patient-reported outcomes (PROs).
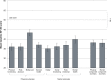
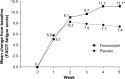
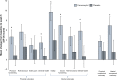
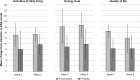
Results: Significantly more patients with IDA receiving ferumoxytol achieved a ≥20 g/L increase in Hgb versus placebo (82.1% vs 1.7%, respectively; P<0.001). Mean increase in Hgb (28.0 g/L vs -1.0 g/L, respectively; P<0.001) significantly favored ferumoxytol treatment. Ferumoxytol-treated patients demonstrated significantly greater improvements than placebo-treated patients relative to their very poor baseline PRO scores posttreatment, including improvements in the Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire and various domains of the 36-Item Short-Form Health Survey. Ferumoxytol-treated patients had a low rate of adverse events.
Conclusion: In this study, ferumoxytol was shown to be an efficacious and generally well-tolerated treatment option for patients with IDA and underlying GI disorders who were unable to use or had a history of unsatisfactory oral iron therapy.
Keywords: efficacy; hemoglobin; inflammatory bowel disease; intravenous iron; patient-reported outcomes; quality of life.
Figures

References
-
- Niv E, Elis A, Zissin R, Naftali T, Novis B, Lishner M. Iron deficiency anemia in patients without gastrointestinal symptoms – a prospective study. Fam Pract. 2005;22(1):58–61. - PubMed
-
- Carter D, Levi G, Tzur D, Novis B, Avidan B. Prevalence and predictive factors for gastrointestinal pathology in young men evaluated for iron deficiency anemia. Dig Dis Sci. 2013;58(5):1299–1305. - PubMed
-
- Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109–116. - PubMed
-
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931–945. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

