Dual Repression of the Multidrug Efflux Pump CmeABC by CosR and CmeR in Campylobacter jejuni
- PMID: 27468281
- PMCID: PMC4943160
- DOI: 10.3389/fmicb.2016.01097
Dual Repression of the Multidrug Efflux Pump CmeABC by CosR and CmeR in Campylobacter jejuni
Abstract
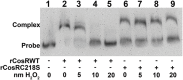
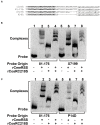
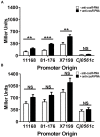
During transmission and intestinal colonization, Campylobacter jejuni, a major foodborne human pathogen, experiences oxidative stress. CosR, a response regulator in C. jejuni, modulates the oxidative stress response and represses expression of the CmeABC multidrug efflux pump. CmeABC, a key component in resistance to toxic compounds including antimicrobials and bile salts, is also under negative regulation by CmeR, a TetR family transcriptional regulator. How CosR and CmeR interact in binding to the cmeABC promoter and how CosR senses oxidative stress are still unknown. To answer these questions, we conducted various experiments utilizing electrophoretic mobility shift assays and transcriptional fusion assays. CosR and CmeR bound independently to two separate sites of the cmeABC promoter, simultaneously repressing cmeABC expression. This dual binding of CosR and CmeR is optimal with a 17 base pair space between the two binding sites as mutations that shortened the distance between the binding sites decreased binding by CmeR and enhanced cmeABC expression. Additionally, the single cysteine residue (C218) of CosR was sensitive to oxidation, which altered the DNA-binding activity of CosR and dissociated CosR from the cmeABC promoter as determined by electrophoretic mobility shift assay. Replacement of C218 with serine rendered CosR insensitive to oxidation, suggesting a potential role of C218 in sensing oxidative stress and providing a possible mechanism for CosR-mediated response to oxidative stress. These findings reveal a dual regulatory role of CosR and CmeR in modulating cmeABC expression and suggest a potential mechanism that may explain overexpression of cmeABC in response to oxidative stress. Differential expression of cmeABC mediated by CmeR and CosR in response to different signals may facilitate adaptation of Campylobacter to various environmental conditions.
Keywords: Campylobacter jejuni; CmeABC; CmeR; CosR; efflux pump; oxidative stress; transcriptional regulation.
Figures
Similar articles
-
Genetic Basis and Functional Consequences of Differential Expression of the CmeABC Efflux Pump in Campylobacter jejuni Isolates.PLoS One. 2015 Jul 1;10(7):e0131534. doi: 10.1371/journal.pone.0131534. eCollection 2015. PLoS One. 2015. PMID: 26132196 Free PMC article.
-
Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni.J Bacteriol. 2005 Nov;187(21):7417-24. doi: 10.1128/JB.187.21.7417-7424.2005. J Bacteriol. 2005. PMID: 16237025 Free PMC article.
-
Transcriptional regulation of the CmeABC multidrug efflux pump and the KatA catalase by CosR in Campylobacter jejuni.J Bacteriol. 2012 Dec;194(24):6883-91. doi: 10.1128/JB.01636-12. Epub 2012 Oct 12. J Bacteriol. 2012. PMID: 23065977 Free PMC article.
-
Structures of AcrR and CmeR: insight into the mechanisms of transcriptional repression and multi-drug recognition in the TetR family of regulators.Biochim Biophys Acta. 2009 May;1794(5):844-51. doi: 10.1016/j.bbapap.2008.12.001. Epub 2008 Dec 14. Biochim Biophys Acta. 2009. PMID: 19130905 Free PMC article. Review.
-
RND-type drug efflux pumps from Gram-negative bacteria: molecular mechanism and inhibition.Front Microbiol. 2015 Apr 28;6:377. doi: 10.3389/fmicb.2015.00377. eCollection 2015. Front Microbiol. 2015. PMID: 25972857 Free PMC article. Review.
Cited by
-
The cost of bacterial predation via type VI secretion system leads to predator extinction under environmental stress.iScience. 2021 Nov 26;24(12):103507. doi: 10.1016/j.isci.2021.103507. eCollection 2021 Dec 17. iScience. 2021. PMID: 34934926 Free PMC article.
-
Investigating the Role of FlhF Identifies Novel Interactions With Genes Involved in Flagellar Synthesis in Campylobacter jejuni.Front Microbiol. 2020 Mar 24;11:460. doi: 10.3389/fmicb.2020.00460. eCollection 2020. Front Microbiol. 2020. PMID: 32265885 Free PMC article.
-
Antimicrobial resistance in enteric bacteria: current state and next-generation solutions.Gut Microbes. 2020 Nov 9;12(1):1799654. doi: 10.1080/19490976.2020.1799654. Gut Microbes. 2020. PMID: 32772817 Free PMC article. Review.
-
Structural basis of DNA recognition of the Campylobacter jejuni CosR regulator.mBio. 2024 Mar 13;15(3):e0343023. doi: 10.1128/mbio.03430-23. Epub 2024 Feb 7. mBio. 2024. PMID: 38323832 Free PMC article.
-
Membrane Proteocomplexome of Campylobacter jejuni Using 2-D Blue Native/SDS-PAGE Combined to Bioinformatics Analysis.Front Microbiol. 2020 Nov 19;11:530906. doi: 10.3389/fmicb.2020.530906. eCollection 2020. Front Microbiol. 2020. PMID: 33329413 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

