Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord
- PMID: 27468657
- PMCID: PMC5035216
- DOI: 10.1016/j.expneurol.2016.07.018
Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord
Abstract
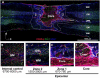
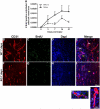
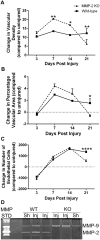
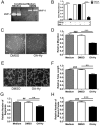
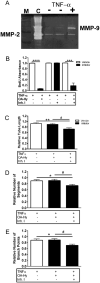
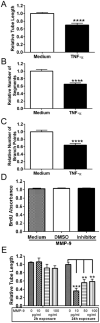
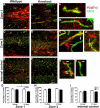
Angiogenesis plays a critical role in wound healing after spinal cord injury. Therefore, understanding the events that regulate angiogenesis has considerable relevance from a therapeutic standpoint. We evaluated the contribution of matrix metalloproteinase (MMP)-2 to angiogenesis and vascular stability in spinal cord injured MMP-2 knockout and wildtype (WT) littermates. While MMP-2 deficiency resulted in reduced endothelial cell division within the lesioned epicenter, there were no genotypic differences in vascularity (vascular density, vascular area, and endothelial cell number) over the first two weeks post-injury. However, by 21days post-injury MMP-2 deficiency resulted in a sharp decline in vascularity, indicative of vascular regression. Complementary in vitro studies of brain capillary endothelial cells confirmed MMP-2 dependent proliferation and tube formation. As deficiency in MMP-2 led to prolonged MMP-9 expression in the injured spinal cord, we examined both short-term and long-term exposure to MMP-9 in vitro. While MMP-9 supported endothelial tube formation and proliferation, prolonged exposure resulted in loss of tubes, findings consistent with vascular regression. Vascular instability is frequently associated with pericyte dissociation and precedes vascular regression. Quantification of PDGFrβ+ pericyte coverage of mature vessels within the glial scar (the reactive gliosis zone), a known source of MMP-9, revealed reduced coverage in MMP-2 deficient animals. These findings suggest that acting in the absence of MMP-2, MMP-9 transiently supports angiogenesis during the early phase of wound healing while its prolonged expression leads to vascular instability and regression. These findings should be considered while developing therapeutic interventions that block MMPs.
Keywords: Angiogenesis; Contusion injury; Matrix metalloproteinases; PDGFrβ positive pericytes; Proliferation; Vascular regression; Vascularity.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Differential cavitation, angiogenesis and wound-healing responses in injured mouse and rat spinal cords.Neuroscience. 2014 Sep 5;275:62-80. doi: 10.1016/j.neuroscience.2014.06.003. Epub 2014 Jun 11. Neuroscience. 2014. PMID: 24929066
-
Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3.J Cell Biol. 2006 Oct 9;175(1):179-91. doi: 10.1083/jcb.200603176. J Cell Biol. 2006. PMID: 17030988 Free PMC article.
-
Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury.Am J Pathol. 2014 Nov;184(11):2985-3000. doi: 10.1016/j.ajpath.2014.07.016. Epub 2014 Oct 14. Am J Pathol. 2014. PMID: 25325922
-
Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury.Neurotherapeutics. 2011 Apr;8(2):206-20. doi: 10.1007/s13311-011-0038-0. Neurotherapeutics. 2011. PMID: 21455784 Free PMC article. Review.
-
Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury.Neurobiol Dis. 2009 Oct;36(1):200-12. doi: 10.1016/j.nbd.2009.07.012. Epub 2009 Jul 23. Neurobiol Dis. 2009. PMID: 19631747
Cited by
-
The Role of Biomaterials as Angiogenic Modulators of Spinal Cord Injury: Mimetics of the Spinal Cord, Cell and Angiogenic Factor Delivery Agents.Front Pharmacol. 2018 Feb 27;9:164. doi: 10.3389/fphar.2018.00164. eCollection 2018. Front Pharmacol. 2018. PMID: 29535633 Free PMC article. Review.
-
Matrix metalloproteinase signals following neurotrauma are right on cue.Cell Mol Life Sci. 2019 Aug;76(16):3141-3156. doi: 10.1007/s00018-019-03176-4. Epub 2019 Jun 6. Cell Mol Life Sci. 2019. PMID: 31168660 Free PMC article. Review.
-
Glial Cells Shape Pathology and Repair After Spinal Cord Injury.Neurotherapeutics. 2018 Jul;15(3):554-577. doi: 10.1007/s13311-018-0630-7. Neurotherapeutics. 2018. PMID: 29728852 Free PMC article. Review.
-
Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences.Int J Mol Sci. 2025 Jul 25;26(15):7184. doi: 10.3390/ijms26157184. Int J Mol Sci. 2025. PMID: 40806316 Free PMC article. Review.
-
Overexpression of MMPs in Corneas Requiring Penetrating and Deep Anterior Lamellar Keratoplasty.Invest Ophthalmol Vis Sci. 2019 Apr 1;60(5):1734-1747. doi: 10.1167/iovs.18-25961. Invest Ophthalmol Vis Sci. 2019. PMID: 31022731 Free PMC article.
References
-
- Ambili M, Sudhakaran PR. 60K gelatinase in involuting rat mammary gland is produced as a 90K proenzyme. Biochemistry and molecular biology international. 1998;45:389–399. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–523. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. - PubMed
-
- Baluk P, Raymond WW, Ator E, Coussens LM, McDonald DM, Caughey GH. Matrix metalloproteinase-2 and -9 expression increases in Mycoplasma-infected airways but is not required for microvascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L307–317. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

