Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts
- PMID: 27468688
- PMCID: PMC4973361
- DOI: 10.1038/cddis.2016.224
Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts
Erratum in
-
Correction: Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts.Cell Death Dis. 2025 Mar 11;16(1):169. doi: 10.1038/s41419-025-07382-w. Cell Death Dis. 2025. PMID: 40069163 Free PMC article. No abstract available.
Abstract
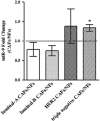
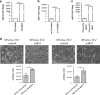
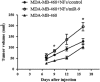
It is established that the interaction between microenvironment and cancer cells has a critical role in tumor development, given the dependence of neoplastic cells on stromal support. However, how this communication promotes the activation of normal (NFs) into cancer-associated fibroblasts (CAFs) is still not well understood. Most microRNA (miRNA) studies focused on tumor cell, but there is increasing evidence of their involvement in reprogramming NFs into CAFs. Here we show that miR-9, upregulated in various breast cancer cell lines and identified as pro-metastatic miRNA, affects the properties of human breast fibroblasts, enhancing the switch to CAF phenotype, thus contributing to tumor growth. Expressed at higher levels in primary triple-negative breast CAFs versus NFs isolated from patients, miR-9 improves indeed migration and invasion capabilities when transfected in immortalized NFs; viceversa, these properties are strongly impaired in CAFs upon miR-9 inhibition. We also demonstrate that tumor-secreted miR-9 can be transferred via exosomes to recipient NFs and this uptake results in enhanced cell motility. Moreover, we observed that this miRNA is also secreted by fibroblasts and in turn able to alter tumor cell behavior, by modulating its direct target E-cadherin, and NFs themselves. Consistently with the biological effects observed, gene expression profiles of NFs upon transient transfection with miR-9 show the modulation of genes mainly involved in cell motility and extracellular matrix remodeling pathways. Finally, we were able to confirm the capability of NFs transiently transfected with miR-9 to promote in vivo tumor growth. Taken together, these data provide new insights into the role of miR-9 as an important player in the cross-talk between cancer cells and stroma.
Figures



References
-
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 2006; 5: 1597–1601. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. - PubMed
-
- Rozenchan PB, Carraro DM, Brentani H, de Carvalho Mota LD, Bastos EP, Ferreira EN et al. Reciprocal changes in gene expression profiles of cocultured breast epithelial cells and primary fibroblasts. Int J Cancer 2009; 125: 2767–2777. - PubMed
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004; 6: 17–32. - PubMed
-
- Casey T, Bond J, Tighe S, Hunter T, Lintault L, Patel O et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res Treat 2009; 114: 47–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

