Regulation of actin dynamics by WNT-5A: implications for human airway smooth muscle contraction
- PMID: 27468699
- PMCID: PMC4965744
- DOI: 10.1038/srep30676
Regulation of actin dynamics by WNT-5A: implications for human airway smooth muscle contraction
Abstract
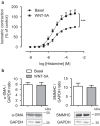
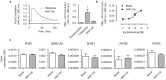
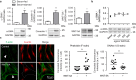
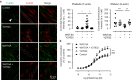
A defining feature of asthma is airway hyperresponsiveness (AHR), which underlies the exaggerated bronchoconstriction response of asthmatics. The role of the airway smooth muscle (ASM) in AHR has garnered increasing interest over the years, but how asthmatic ASM differs from healthy ASM is still an active topic of debate. WNT-5A is increasingly expressed in asthmatic ASM and has been linked with Th2-high asthma. Due to its link with calcium and cytoskeletal remodelling, we propose that WNT-5A may modulate ASM contractility. We demonstrated that WNT-5A can increase maximum isometric tension in bovine tracheal smooth muscle strips. In addition, we show that WNT-5A is preferentially expressed in contractile human airway myocytes compared to proliferative cells, suggesting an active role in maintaining contractility. Furthermore, WNT-5A treatment drives actin polymerisation, but has no effect on intracellular calcium flux. Next, we demonstrated that WNT-5A directly regulates TGF-β1-induced expression of α-SMA via ROCK-mediated actin polymerization. These findings suggest that WNT-5A modulates fundamental mechanisms that affect ASM contraction and thus may be of relevance for AHR in asthma.
Figures

References
-
- WHO. Asthma. Available at: http://www.who.int/respiratory/asthma/en/ (2013).
-
- Bai T. R. Abnormalities in airway smooth muscle in fatal asthma. A comparison between trachea and bronchus. Am. Rev. Respir. Dis. 143, 441–443 (1991). - PubMed
-
- Cerrina J. et al. Human isolated bronchial muscle preparations from asthmatic patients: effects of indomethacin and contractile agonists. Prostaglandins 37, 457–469 (1989). - PubMed
-
- Chin L. Y. M. et al. Mechanical properties of asthmatic airway smooth muscle. Eur. Respir. J. 40, 45–54 (2012). - PubMed
-
- Ma X. et al. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L1181–1189 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

