In vivo monitoring of lung inflammation in CFTR-deficient mice
- PMID: 27468800
- PMCID: PMC4964274
- DOI: 10.1186/s12967-016-0976-8
In vivo monitoring of lung inflammation in CFTR-deficient mice
Abstract
Background: Experimentally, lung inflammation in laboratory animals is usually detected by the presence of inflammatory markers, such as immune cells and cytokines, in the bronchoalveolar lavage fluid (BALF) of sacrificed animals. This method, although extensively used, is time, money and animal life consuming, especially when applied to genetically modified animals. Thus a new and more convenient approach, based on in vivo imaging analysis, has been set up to evaluate the inflammatory response in the lung of CFTR-deficient (CF) mice, a murine model of cystic fibrosis.
Methods: Wild type (WT) and CF mice were stimulated with P. aeruginosa LPS, TNF-alpha and culture supernatant derived from P. aeruginosa (strain VR1). Lung inflammation was detected by measuring bioluminescence in vivo in mice transiently transgenized with a luciferase reporter gene under the control of a bovine IL-8 gene promoter.
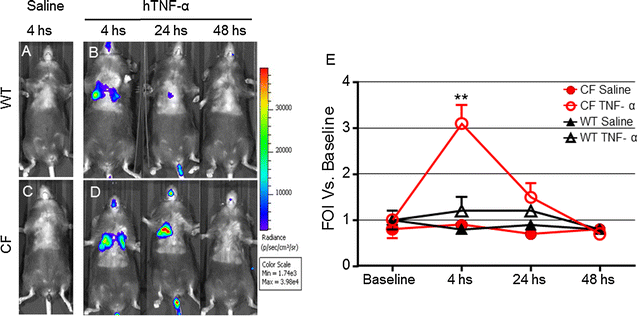
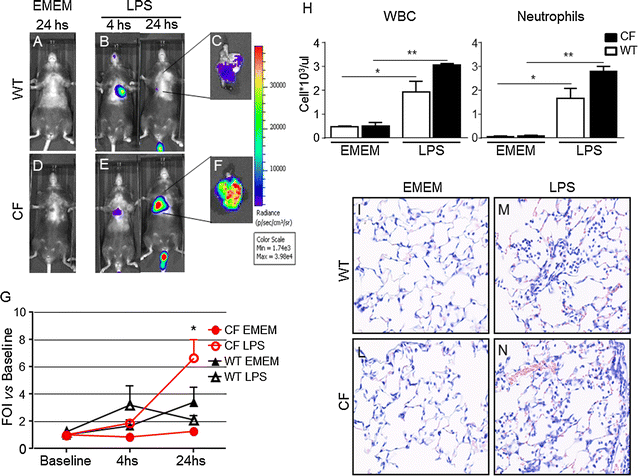
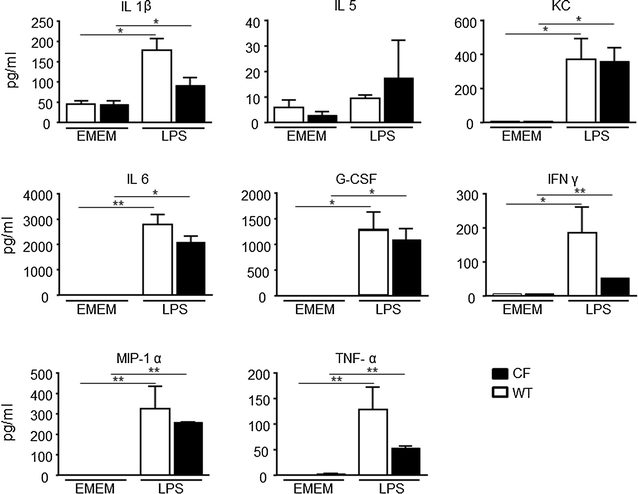
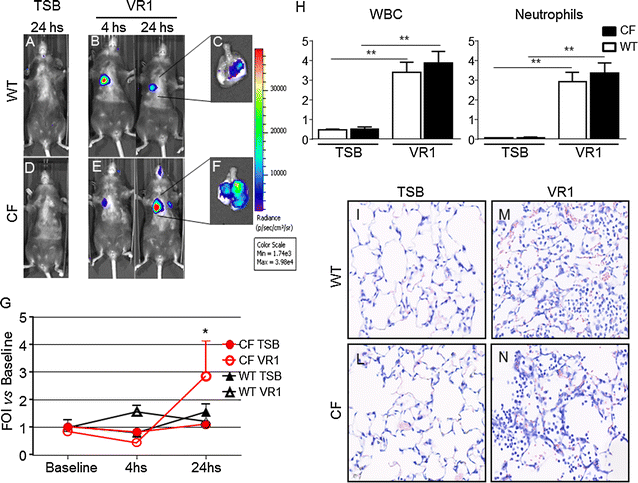
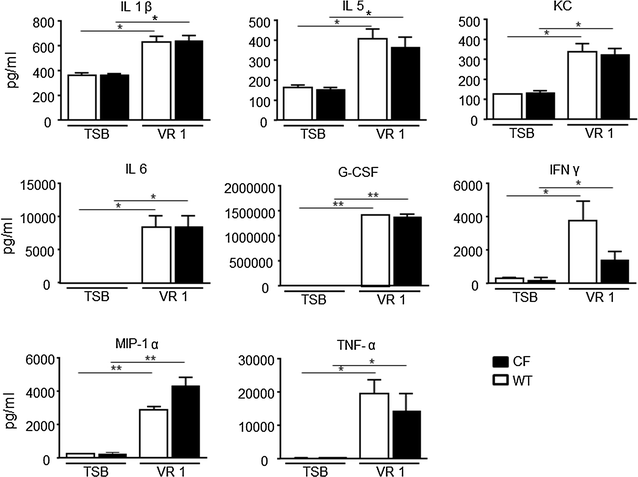
Results: Differences in bioluminescence (BLI) signal were revealed by comparing the two types of mice after intratracheal challenge with pro-inflammatory stimuli. BLI increased at 4 h after stimulation with TNF-alpha and at 24 h after administration of LPS and VR1 supernatant in CF mice with respect to untreated animals. The BLI signal was significantly more intense and lasted for longer times in CF animals when compared to WT mice. Analysis of BALF markers: leukocytes, cytokines and histology revealed no significant differences between CF and WT mice.
Conclusions: In vivo gene delivery technology and non-invasive bioluminescent imaging has been successfully adapted to CFTR-deficient mice. Activation of bIL-8 transgene promoter can be monitored by non-invasive BLI imaging in the lung of the same animal and compared longitudinally in both CF or WT mice, after challenge with pro-inflammatory stimuli. The combination of these technologies and the use of CF mice offer the unique opportunity of evaluating the impact of therapies aimed to control inflammation in a CF background.
Keywords: CFTR-deficient mice; In vivo bioluminescence imaging; Lung inflammation mouse model; Pseudomonas aeruginosa.
Figures
References
-
- Galluzzo M, Ciraolo E, Lucattelli M, Hoxha E, Ulrich M, Campa CC, et al. Genetic deletion and pharmacological inhibition of PI3 K γ reduces neutrophilic airway inflammation and lung damage in mice with cystic fibrosis-like lung disease. Mediators Inflamm. 2015;2015:545417. doi: 10.1155/2015/545417. - DOI - PMC - PubMed
-
- Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, et al. Mutations of cystic fibrosis transmembrane conductance regulator (CFTR) gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med. 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

