The effects of lymph node status on predicting outcome in ER+ /HER2- tamoxifen treated breast cancer patients using gene signatures
- PMID: 27469239
- PMCID: PMC4964078
- DOI: 10.1186/s12885-016-2501-0
The effects of lymph node status on predicting outcome in ER+ /HER2- tamoxifen treated breast cancer patients using gene signatures
Abstract
Background: Lymph node (LN) status is the most important prognostic variable used to guide ER positive (+) breast cancer treatment. While a positive nodal status is traditionally associated with a poor prognosis, a subset of these patients respond well to treatment and achieve long-term survival. Several gene signatures have been established as a means of predicting outcome of breast cancer patients, but the development and indication for use of these assays varies. Here we compare the capacity of two approved gene signatures and a third novel signature to predict outcome in distinct LN negative (-) and LN+ populations. We also examine biological differences between tumours associated with LN- and LN+ disease.
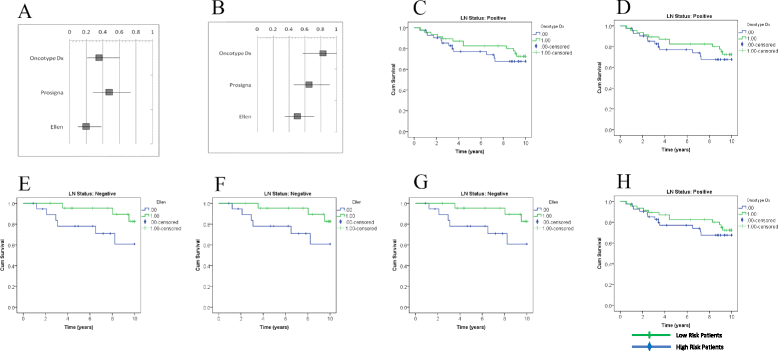
Methods: Gene expression data from publically available data sets was used to compare the ability of Oncotype DX and Prosigna to predict Distant Metastasis Free Survival (DMFS) using an in silico platform. A novel gene signature (Ellen) was developed by including patients with both LN- and LN+ disease and using Prediction Analysis of Microarrays (PAM) software. Gene Set Enrichment Analysis (GSEA) was used to determine biological pathways associated with patient outcome in both LN- and LN+ tumors.
Results: The Oncotype DX gene signature, which only used LN- patients during development, significantly predicted outcome in LN- patients, but not LN+ patients. The Prosigna gene signature, which included both LN- and LN+ patients during development, predicted outcome in both LN- and LN+ patient groups. Ellen was also able to predict outcome in both LN- and LN+ patient groups. GSEA suggested that epigenetic modification may be related to poor outcome in LN- disease, whereas immune response may be related to good outcome in LN+ disease.
Conclusions: We demonstrate the importance of incorporating lymph node status during the development of prognostic gene signatures. Ellen may be a useful tool to predict outcome of patients regardless of lymph node status, or for those with unknown lymph node status. Finally we present candidate biological processes, unique to LN- and LN+ disease, that may indicate risk of relapse.
Keywords: Breast cancer; Estrogen receptor; Gene signature; Lymph node status; Oncotype DX; Prognosis; Prosigna.
Figures

References
-
- CCO. Surgical Management of Early-Stage Invasive Breast Cancer Overview Guideline Report History. 2011.
-
- NCCN . Practice Guidelines in Oncology. 2012.
-
- Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, Bryant J, Dimitrov NV, Abramson N, Atkins JN, Shibata H, Deschenes L, Margolese RG. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. - DOI - PubMed
-
- Capulli M, Angelucci A, Driouch K, Garcia T, Clement-Lacroix P, Martella F, Ventura L, Bologna M, Flamini S, Moreschini O, Lidereau R, Ricevuto E, Muraca M, Teti A, Rucci N. Increased expression of a set of genes enriched in oxygen binding function discloses a predisposition of breast cancer bone metastases to generate metastasis spread in multiple organs. J Bone Miner Res. 2012;27:2387–2398. doi: 10.1002/jbmr.1686. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

