A small-angle X-ray scattering study of alpha-synuclein from human red blood cells
- PMID: 27469540
- PMCID: PMC4965831
- DOI: 10.1038/srep30473
A small-angle X-ray scattering study of alpha-synuclein from human red blood cells
Abstract
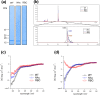
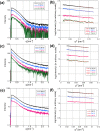
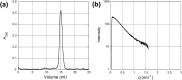
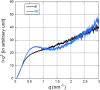
α-synuclein (α-syn) is the main component of Lewy bodies, which are neuropathological hallmarks of patients with Parkinson's disease. As it has been controversial whether human α-syn from erythrocytes exists as a tetramer under physiological conditions, we tried solving this issue by the small-angle X-ray solution scattering method. Under two different conditions (high ionic strength with a Tris buffer and low ionic strength with an ammonium acetate buffer), no evidence was found for the presence of tetramer. When comparing erythrocyte and recombinant α-syn molecules, we found no significant difference of the molecular weight and the secondary structure although the buffer conditions strongly affect the radius of gyration of the protein. The results indicate that, even though a stable tetramer may not be formed, conformation of α-syn depends much on its environment, which may be the reason for its tendency to aggregate in cells.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

