Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care
- PMID: 27470089
- PMCID: PMC4974573
- DOI: 10.1038/ncomms12211
Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care
Abstract
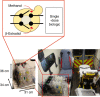
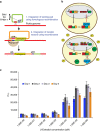
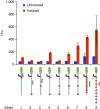
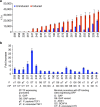
Current biopharmaceutical manufacturing systems are not compatible with portable or distributed production of biologics, as they typically require the development of single biologic-producing cell lines followed by their cultivation at very large scales. Therefore, it remains challenging to treat patients in short time frames, especially in remote locations with limited infrastructure. To overcome these barriers, we developed a platform using genetically engineered Pichia pastoris strains designed to secrete multiple proteins on programmable cues in an integrated, benchtop, millilitre-scale microfluidic device. We use this platform for rapid and switchable production of two biologics from a single yeast strain as specified by the operator. Our results demonstrate selectable and near-single-dose production of these biologics in <24 h with limited infrastructure requirements. We envision that combining this system with analytical, purification and polishing technologies could lead to a small-scale, portable and fully integrated personal biomanufacturing platform that could advance disease treatment at point-of-care.
Figures


References
-
- Thiel K. A. Biomanufacturing, from bust to boom...to bubble? Nat. Biotechnol. 22, 1365–1372 (2004). - PubMed
-
- Dove A. Uncorking the biomanufacturing bottleneck. Nat. Biotechnol. 20, 777–779 (2002). - PubMed
-
- Gottschalk U., Brorson K. & Shukla A. A. The need for innovation in biomanufacturing. Nat. Biotechnol. 30, 489–492 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

