Incompatibility of lyophilized inactivated polio vaccine with liquid pentavalent whole-cell-pertussis-containing vaccine
- PMID: 27470209
- PMCID: PMC5009896
- DOI: 10.1016/j.vaccine.2016.07.030
Incompatibility of lyophilized inactivated polio vaccine with liquid pentavalent whole-cell-pertussis-containing vaccine
Abstract
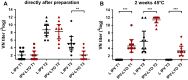
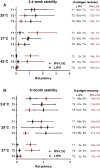
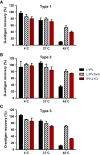
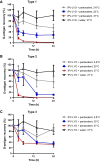
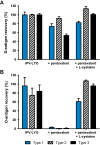
A hexavalent vaccine containing diphtheria toxoid, tetanus toxoid, whole cell pertussis, Haemophilius influenza type B, hepatitis B and inactivated polio vaccine (IPV) may: (i) increase the efficiency of vaccination campaigns, (ii) reduce the number of injections thereby reducing needlestick injuries, and (iii) ensure better protection against pertussis as compared to vaccines containing acellular pertussis antigens. An approach to obtain a hexavalent vaccine might be reconstituting lyophilized polio vaccine (IPV-LYO) with liquid pentavalent vaccine just before intramuscular delivery. The potential limitations of this approach were investigated including thermostability of IPV as measured by D-antigen ELISA and rat potency, the compatibility of fluid and lyophilized IPV in combination with thimerosal and thimerosal containing hexavalent vaccine. The rat potency of polio type 3 in IPV-LYO was 2 to 3-fold lower than standardized on the D-antigen content, suggesting an alteration of the polio type 3 D-antigen particle by lyophilization. Type 1 and 2 had unaffected antigenicity/immunogenicity ratios. Alteration of type 3 D-antigen could be detected by showing reduced thermostability at 45°C compared to type 3 in non-lyophilized liquid controls. Reconstituting IPV-LYO in the presence of thimerosal (TM) resulted in a fast temperature dependent loss of polio type 1-3 D-antigen. The presence of 0.005% TM reduced the D-antigen content by ∼20% (polio type 2/3) and ∼60% (polio type 1) in 6h at 25°C, which are WHO open vial policy conditions. At 37°C, D-antigen was diminished even faster, suggesting that very fast, i.e., immediately after preparation, intramuscular delivery of the conceived hexavalent vaccine would not be a feasible option. Use of the TM-scavenger, l-cysteine, to bind TM (or mercury containing TM degradation products), resulted in a hexavalent vaccine mixture in which polio D-antigen was more stable.
Keywords: Hexavalent vaccine; Inactivated polio vaccine; Lyophilization; Rat potency; Thimerosal.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Safety and immunogenicity of a toddler dose following an infant series of a hexavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, hepatitis B vaccine administered concurrently or at separate visits with a heptavalent pneumococcal conjugate vaccine.Pediatr Infect Dis J. 2014 Jan;33(1):73-80. doi: 10.1097/01.inf.0000437806.76221.20. Pediatr Infect Dis J. 2014. PMID: 24346596 Clinical Trial.
-
Immunological persistence in 5 y olds previously vaccinated with hexavalent DTPa-HBV-IPV/Hib at 3, 5, and 11 months of age.Hum Vaccin Immunother. 2014;10(10):2795-8. doi: 10.4161/21645515.2014.970494. Hum Vaccin Immunother. 2014. PMID: 25483640 Free PMC article.
-
Safety and immunogenicity of three different formulations of a liquid hexavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae b conjugate-hepatitis B vaccine at 2, 4, 6 and 12-14 months of age.Vaccine. 2011 Feb 1;29(6):1324-31. doi: 10.1016/j.vaccine.2010.11.053. Epub 2010 Dec 4. Vaccine. 2011. PMID: 21134456 Clinical Trial.
-
New combination vaccines: DTaP-IPV (Kinrix) and DTaP-IPV/Hib (Pentacel).Ann Pharmacother. 2010 Mar;44(3):515-23. doi: 10.1345/aph.1M468. Ann Pharmacother. 2010. PMID: 20197476 Review.
-
Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa).BioDrugs. 2010 Oct 1;24(5):299-302. doi: 10.2165/11206690-000000000-00000. BioDrugs. 2010. PMID: 20795752 Review.
Cited by
-
Evaluating the Compatibility of New Recombinant Protein Antigens (Trivalent NRRV) with a Mock Pentavalent Combination Vaccine Containing Whole-Cell Pertussis: Analytical and Formulation Challenges.Vaccines (Basel). 2024 Jun 3;12(6):609. doi: 10.3390/vaccines12060609. Vaccines (Basel). 2024. PMID: 38932338 Free PMC article.
-
Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication.Hum Vaccin Immunother. 2022 Dec 30;18(7):2154100. doi: 10.1080/21645515.2022.2154100. Epub 2022 Dec 28. Hum Vaccin Immunother. 2022. PMID: 36576132 Free PMC article. Review.
-
Capsid destabilization and epitope alterations of human papillomavirus 18 in the presence of thimerosal.J Pharm Anal. 2021 Oct;11(5):617-627. doi: 10.1016/j.jpha.2020.08.007. Epub 2020 Aug 28. J Pharm Anal. 2021. PMID: 34765275 Free PMC article.
References
-
- Sawyer L.A., McInnis J., Patel A., Horne A.D., Albrecht P. Deleterious effect of thimerosal on the potency of inactivated poliovirus vaccine. Vaccine. 1994;12:851–856. - PubMed
-
- Recommendations for whole-cell pertussis vaccine. Technical report series 941. World Health Organisation; 2007.
-
- Thomassen Y.E., van Sprang E.N., van der Pol L.A., Bakker W.A. Multivariate data analysis on historical IPV production data for better process understanding and future improvements. Biotechnol Bioeng. 2010;107:96–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

