Early Height and Weight Changes in Children Using Cotrimoxazole Prophylaxis With Antiretroviral Therapy
- PMID: 27470239
- PMCID: PMC5064162
- DOI: 10.1093/cid/ciw514
Early Height and Weight Changes in Children Using Cotrimoxazole Prophylaxis With Antiretroviral Therapy
Abstract
Background: The growth benefits of cotrimoxazole during early antiretroviral therapy (ART) are not well characterized.
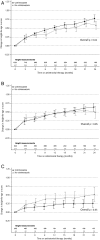
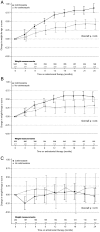
Methods: Individuals enrolled in the Therapeutics Research, Education, and AIDS Training in Asia Pediatric HIV Observational Database were included if they started ART at ages 1 month-14 years and had both height and weight measurements available at ART initiation (baseline). Generalized estimating equations were used to identify factors associated with change in height-for-age z-score (HAZ), follow-up HAZ ≥ -2, change in weight-for-age z-score (WAZ), and follow-up WAZ ≥ -2.
Results: A total of 3217 children were eligible for analysis. The adjusted mean change in HAZ among cotrimoxazole and non-cotrimoxazole users did not differ significantly over the first 24 months of ART. In children who were stunted (HAZ < -2) at baseline, cotrimoxazole use was not associated with a follow-up HAZ ≥ -2. The adjusted mean change in WAZ among children with a baseline CD4 percentage (CD4%) >25% became significantly different between cotrimoxazole and non-cotrimoxazole users after 6 months of ART and remained significant after 24 months (overall P < .01). Similar changes in WAZ were observed in those with a baseline CD4% between 10% and 24% (overall P < .01). Cotrimoxazole use was not associated with a significant difference in follow-up WAZ in children with a baseline CD4% <10%. In those underweight (WAZ < -2) at baseline, cotrimoxazole use was associated with a follow-up WAZ ≥ -2 (adjusted odds ratio, 1.70 vs not using cotrimoxazole [95% confidence interval, 1.28-2.25], P < .01). This association was driven by children with a baseline CD4% ≥10%.
Conclusions: Cotrimoxazole use is associated with benefits to WAZ but not HAZ during early ART in Asian children.
Keywords: antiretroviral therapy; children; cotrimoxazole; height; weight.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
References
-
- World Health Organization, ART Guidelines Committee. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach. December 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_dec2014/en/ Accessed 20 January 2016. - PubMed
-
- World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach—2010 revision. Available at http://www.who.int/hiv/pub/paediatric/infants2010/en/ Accessed 19 February 2014. - PubMed
-
- World Health Organization, ART Guidelines Committee. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach—June 2013. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 Accessed 3 January 2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

