Randomized controlled trial of supportive-expressive group therapy and body-mind-spirit intervention for Chinese non-metastatic breast cancer patients
- PMID: 27470259
- PMCID: PMC5082591
- DOI: 10.1007/s00520-016-3350-8
Randomized controlled trial of supportive-expressive group therapy and body-mind-spirit intervention for Chinese non-metastatic breast cancer patients
Abstract
Purpose: This study aimed to evaluate the efficacy of supportive-expressive group (SEG) therapy and body-mind-spirit (BMS) intervention on emotional suppression and psychological distress in Chinese breast cancer patients.
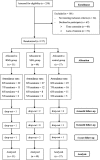
Methods: This three-arm randomized controlled trial assigned 157 non-metastatic breast cancer patients to BMS, SEG, or social support control group. SEG focused on emotional expression and group support, whereas BMS emphasized relaxation and self-care. All groups received 2-h weekly sessions for 8 weeks. The participants completed measurements on emotional suppression, perceived stress, anxiety, and depression at baseline and three follow-up assessments in 1 year.
Results: Using latent growth modeling, overall group difference was found for emotional suppression (χ 2(2) = 8.88, p = 0.012), marginally for perceived stress (χ 2(2) = 5.70, p = 0.058), but not for anxiety and depression (χ 2(2) = 0.19-0.94, p > 0.05). Post-hoc analyses revealed a significant and moderate reduction (Cohen d = 0.55, p = 0.007) in emotional suppression in SEG compared to control group, whereas BMS resulted in a marginally significant and moderate fall (d = 0.46, p = 0.024) in perceived stress. Neither SEG nor BMS significantly improved anxiety and depression (d < 0.20, p > 0.05).
Conclusions: The present results did not demonstrate overall effectiveness for either BMS or SEG therapy in the present sample of Chinese non-metastatic breast cancer patients. The participants appear to derive only modest benefits in terms of their psychological well-being from either intervention.
Keywords: Body-mind-spirit; Breast cancer; Chinese; Emotional suppression; Psychological distress; Supportive-expressive therapy.
Conflict of interest statement
Compliance with ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Conflict of interest The authors declare that they have no conflict of interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical