Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation
- PMID: 27470424
- PMCID: PMC5006729
- DOI: 10.1016/j.ebiom.2016.07.021
Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation
Abstract
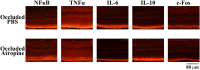
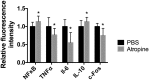
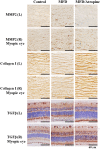
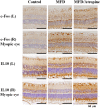
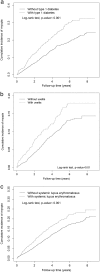
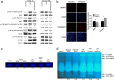
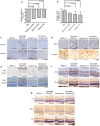
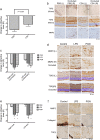
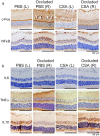
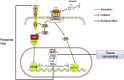
Prevention and treatment of myopia is an important public problem worldwide. We found a higher incidence of myopia among patients with inflammatory diseases such as type 1 diabetes mellitus (7.9%), uveitis (3.7%), or systemic lupus erythematosus (3.5%) compared to those without inflammatory diseases (p<0.001) using data from children (<18years old) in the National Health Insurance Research database. We then examined the inhibition of myopia by atropine in Syrian hamsters with monocular form deprivation (MFD), an experimental myopia model. We found atropine downregulated inflammation in MFD eyes. The expression levels of c-Fos, nuclear factor κB (NFκB), interleukin (IL)-6, and tumor necrosis factor (TNF)-α were upregulated in myopic eyes and downregulated upon treatment with atropine. The relationship between the inflammatory response and myopia was investigated by treating MFD hamsters with the immunosuppressive agent cyclosporine A (CSA) or the inflammatory stimulators lipopolysaccharide (LPS) or peptidoglycan (PGN). Myopia progression was slowed by CSA application but was enhanced by LPS and PGN administration. The levels of c-Fos, NF-κB, IL-6, and TNF-α were upregulated in LPS- and PGN-treated eyes and downregulated by CSA treatment. These findings provide clinical and experimental evidence that inflammation plays a crucial role in the development of myopia.
Keywords: Inflammation; Interleukin 6; Myopia; Nuclear factor κB; Tumor necrosis factor alpha; c-Fos.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Fallopia Japonica and Prunella vulgaris inhibit myopia progression by suppressing AKT and NFκB mediated inflammatory reactions.BMC Complement Med Ther. 2022 Oct 14;22(1):271. doi: 10.1186/s12906-022-03747-2. BMC Complement Med Ther. 2022. PMID: 36242032 Free PMC article.
-
Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression.EBioMedicine. 2018 Feb;28:274-286. doi: 10.1016/j.ebiom.2018.01.024. Epub 2018 Jan 31. EBioMedicine. 2018. PMID: 29398596 Free PMC article.
-
Crosstalk between Myopia and Inflammation: A Mini Review.Int J Med Sci. 2024 Jun 3;21(9):1589-1603. doi: 10.7150/ijms.94826. eCollection 2024. Int J Med Sci. 2024. PMID: 39006849 Free PMC article. Review.
-
Diacerein Inhibits Myopia Progression through Lowering Inflammation in Retinal Pigment Epithelial Cell.Mediators Inflamm. 2021 Jul 3;2021:6660640. doi: 10.1155/2021/6660640. eCollection 2021. Mediators Inflamm. 2021. PMID: 34285659 Free PMC article.
-
Use of Atropine for Prevention of Childhood Myopia Progression in Clinical Practice.Eye Contact Lens. 2016 Jan;42(1):16-23. doi: 10.1097/ICL.0000000000000189. Eye Contact Lens. 2016. PMID: 26340385 Review.
Cited by
-
Correlation of axial length and myopic macular degeneration to levels of molecular factors in the aqueous.Sci Rep. 2019 Oct 31;9(1):15708. doi: 10.1038/s41598-019-52156-y. Sci Rep. 2019. PMID: 31673022 Free PMC article.
-
Inflammatory cytokines in highly myopic eyes.Sci Rep. 2019 Mar 5;9(1):3517. doi: 10.1038/s41598-019-39652-x. Sci Rep. 2019. PMID: 30837544 Free PMC article.
-
Unveiling the gut microbiota and metabolite profiles in guinea pigs with form deprivation myopia through 16S rRNA gene sequencing and untargeted metabolomics.Heliyon. 2024 May 6;10(9):e30491. doi: 10.1016/j.heliyon.2024.e30491. eCollection 2024 May 15. Heliyon. 2024. PMID: 38756593 Free PMC article.
-
Visually induced changes in cytokine production in the chick choroid.Elife. 2021 Oct 5;10:e70608. doi: 10.7554/eLife.70608. Elife. 2021. PMID: 34608867 Free PMC article.
-
Kawasaki Disease Increases the Incidence of Myopia.Biomed Res Int. 2017;2017:2657913. doi: 10.1155/2017/2657913. Epub 2017 Jul 30. Biomed Res Int. 2017. PMID: 28828383 Free PMC article.
References
-
- Ayazi S., Armstrong W.L., Weinstein A. Transient high myopia in systemic lupus erythematosus associated with anasarca. Ann. Ophthalmol. 1982;14:785–787. - PubMed
-
- Boone M.I., Moore T.L., Cruz O.A. Screening for uveitis in juvenile rheumatoid arthritis. J. Pediatr. Ophthalmol. Strabismus. 1998;35:41–43. - PubMed
-
- Buhling F., Lieder N., Kuhlmann U.C., Waldburg N., Welte T. Tiotropium suppresses acetylcholine-induced release of chemotactic mediators in vitro. Respir. Med. 2007;101:2386–2394. - PubMed
-
- Chen S.J., Tung T.H., Liu J.H., Lee A.F., Lee F.L., Hsu W.M., Chou P. Prevalence and associated factors of refractive errors among type 2 diabetics in Kinmen, Taiwan. Ophthalmic Epidemiol. 2008;15:2–9. - PubMed
-
- Chen K.C., Hsi E., Hu C.Y., Chou W.W., Liang C.L., Juo S.H. MicroRNA-328 may influence myopia development by mediating the PAX6 gene. Invest. Ophthalmol. Vis. Sci. 2012;53:2732–2739. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

