Rapid Brownian Motion Primes Ultrafast Reconstruction of Intrinsically Disordered Phe-Gly Repeats Inside the Nuclear Pore Complex
- PMID: 27470900
- PMCID: PMC4965864
- DOI: 10.1038/srep29991
Rapid Brownian Motion Primes Ultrafast Reconstruction of Intrinsically Disordered Phe-Gly Repeats Inside the Nuclear Pore Complex
Abstract
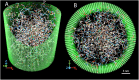
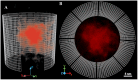
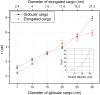
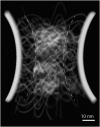
Conformational behavior of intrinsically disordered proteins, such as Phe-Gly repeat domains, alters drastically when they are confined in, and tethered to, nan channels. This has challenged our understanding of how they serve to selectively facilitate translocation of nuclear transport receptor (NTR)-bearing macromolecules. Heterogeneous FG-repeats, tethered to the NPC interior, nonuniformly fill the channel in a diameter-dependent manner and adopt a rapid Brownian motion, thereby forming a porous and highly dynamic polymeric meshwork that percolates in radial and axial directions and features two distinguishable zones: a dense hydrophobic rod-like zone located in the center, and a peripheral low-density shell-like zone. The FG-meshwork is locally disrupted upon interacting with NTR-bearing macromolecules, but immediately reconstructs itself between 0.44 μs and 7.0 μs, depending on cargo size and shape. This confers a perpetually-sealed state to the NPC, and is solely due to rapid Brownian motion of FG-repeats, not FG-repeat hydrophobic bonds. Elongated-shaped macromolecules, both in the presence and absence of NTRs, penetrate more readily into the FG-meshwork compared to their globular counterparts of identical volume and surface chemistry, highlighting the importance of the shape effects in nucleocytoplasmic transport. These results can help our understanding of geometrical effects in, and the design of, intelligent and responsive biopolymer-based materials in nanofiltration and artificial nanopores.
Figures



Similar articles
-
Nucleoporins' exclusive amino acid sequence features regulate their transient interaction with and selectivity of cargo complexes in the nuclear pore.Mol Biol Cell. 2021 Nov 1;32(21):ar31. doi: 10.1091/mbc.E21-04-0161. Epub 2021 Sep 2. Mol Biol Cell. 2021. PMID: 34473567 Free PMC article.
-
Converging on the function of intrinsically disordered nucleoporins in the nuclear pore complex.Biol Chem. 2010 Jul;391(7):719-30. doi: 10.1515/BC.2010.092. Biol Chem. 2010. PMID: 20482319 Review.
-
Brownian dynamics simulation of nucleocytoplasmic transport: a coarse-grained model for the functional state of the nuclear pore complex.PLoS Comput Biol. 2011 Jun;7(6):e1002049. doi: 10.1371/journal.pcbi.1002049. Epub 2011 Jun 2. PLoS Comput Biol. 2011. PMID: 21673865 Free PMC article.
-
Nucleoporin's Like Charge Regions Are Major Regulators of FG Coverage and Dynamics Inside the Nuclear Pore Complex.PLoS One. 2015 Dec 11;10(12):e0143745. doi: 10.1371/journal.pone.0143745. eCollection 2015. PLoS One. 2015. PMID: 26658558 Free PMC article.
-
Biomechanics of the transport barrier in the nuclear pore complex.Semin Cell Dev Biol. 2017 Aug;68:42-51. doi: 10.1016/j.semcdb.2017.05.007. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506890 Review.
Cited by
-
The Role of Cohesiveness in the Permeability of the Spatial Assemblies of FG Nucleoporins.Biophys J. 2019 Apr 2;116(7):1204-1215. doi: 10.1016/j.bpj.2019.02.028. Epub 2019 Mar 7. Biophys J. 2019. PMID: 30902367 Free PMC article.
-
Regulating transport efficiency through the nuclear pore complex: The role of binding affinity with FG-Nups.Mol Biol Cell. 2024 Dec 1;35(12):ar149. doi: 10.1091/mbc.E24-05-0224. Epub 2024 Oct 30. Mol Biol Cell. 2024. PMID: 39475712 Free PMC article.
-
Percolation transition prescribes protein size-specific barrier to passive transport through the nuclear pore complex.Nat Commun. 2022 Sep 1;13(1):5138. doi: 10.1038/s41467-022-32857-1. Nat Commun. 2022. PMID: 36050301 Free PMC article.
-
Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
-
Role of pore dilation in molecular transport through the nuclear pore complex: Insights from polymer scaling theory.PLoS Comput Biol. 2025 Apr 7;21(4):e1012909. doi: 10.1371/journal.pcbi.1012909. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40193850 Free PMC article.
References
-
- Tompa P. Intrinsically unstructured proteins. TRENDS in Biochemical Sciences 27, 527–533 (2002). - PubMed
-
- Babu M. M., Kriwacki R. W. & Pappu R. V. Versatility from protein disorder. Science 337, 1460–1461 (2012). - PubMed
-
- Bah A. et al. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 519, 106–109 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

