PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors
- PMID: 27470968
- PMCID: PMC5241182
- DOI: 10.1158/1078-0432.CCR-16-1163
PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors
Abstract
Purpose: Tyrosine kinase inhibitors are effective in gastrointestinal stromal tumors (GISTs) but often are of transient benefit as resistance commonly develops. Immunotherapy, particularly blockade of the inhibitory receptor programmed death 1 (PD-1) or the ligand programmed death ligand 1 (PD-L1), has shown effectiveness in a variety of cancers. The functional effects of PD-1/PD-L1 blockade are unknown in GISTs.
Experimental design: We analyzed tumor and matched blood samples from 85 patients with GISTs and determined the expression of immune checkpoint molecules using flow cytometry. We investigated the combination of imatinib with PD-1/PD-L1 blockade in KitV558Δ/+ mice that develop GISTs.
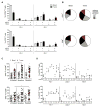
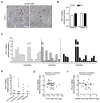
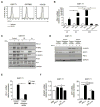
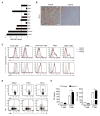
Results: The inhibitory receptors PD-1, lymphocyte activation gene 3, and T-cell immunoglobulin mucin-3 were upregulated on tumor-infiltrating T cells compared with T cells from matched blood. PD-1 expression on T cells was highest in imatinib-treated human GISTs. Meanwhile, intratumoral PD-L1 expression was variable. In human GIST cell lines, treatment with imatinib abrogated the IFNγ-induced upregulation of PD-L1 via STAT1 inhibition. In KitV558Δ/+ mice, imatinib downregulated IFNγ-related genes and reduced PD-L1 expression on tumor cells. PD-1 and PD-L1 blockade in vivo each had no efficacy alone but enhanced the antitumor effects of imatinib by increasing T-cell effector function in the presence of KIT and IDO inhibition.
Conclusions: PD-1/PD-L1 blockade is a promising strategy to improve the effects of targeted therapy in GISTs. Collectively, our results provide the rationale to combine these agents in human GISTs. Clin Cancer Res; 23(2); 454-65. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

References
-
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. - PubMed
-
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. - PubMed
-
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32. - PubMed
-
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

