DNA binding proteins explore multiple local configurations during docking via rapid rebinding
- PMID: 27471033
- PMCID: PMC5041478
- DOI: 10.1093/nar/gkw666
DNA binding proteins explore multiple local configurations during docking via rapid rebinding
Abstract
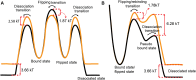
Finding the target site and associating in a specific orientation are essential tasks for DNA-binding proteins. In order to make the target search process as efficient as possible, proteins should not only rapidly diffuse to the target site but also dynamically explore multiple local configurations before diffusing away. Protein flipping is an example of this second process that has been observed previously, but the underlying mechanism of flipping remains unclear. Here, we probed the mechanism of protein flipping at the single molecule level, using HIV-1 reverse transcriptase (RT) as a model system. In order to test the effects of long-range attractive forces on flipping efficiency, we varied the salt concentration and macromolecular crowding conditions. As expected, increased salt concentrations weaken the binding of RT to DNA while increased crowding strengthens the binding. Moreover, when we analyzed the flipping kinetics, i.e. the rate and probability of flipping, at each condition we found that flipping was more efficient when RT bound more strongly. Our data are consistent with a view that DNA bound proteins undergo multiple rapid re-binding events, or short hops, that allow the protein to explore other configurations without completely dissociating from the DNA.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Berg O.G., Winter R.B., Von Hippel P.H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–6948. - PubMed
-
- Berg O.G., von Hippel P.H. Diffusion-Controlled Macromolecular Interactions. Annu. Rev. Biophys. Biophys. Chem. 1985;14:131–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

