miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas
- PMID: 27471108
- PMCID: PMC5193021
- DOI: 10.1093/neuonc/now129
miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas
Abstract
Background: Gliomas are based on a genetic abnormality and present with a dismal prognosis. MicroRNAs (miRNAs) are considered to be important mediators of gene expression in glioma tissues.
Methods: Real-time PCR was used to analyze the expression of microRNA-423-5p (miR-423-5p) in human glioma samples and normal brain tissue. Apoptosis, cell cycle, proliferation, immunostaining, transwell, in vitro 2D and 3D migration, and chemosensitivity assays were performed to assess the phenotypic changes in glioma cells overexpressing miRNA-423-5p. Western blotting was used to determine the expression of inhibitor of growth 4 (ING-4)in glioma tissues, and a luciferase reporter assay was conducted to confirm whether ING-4 is a direct target of miR-423-5p. Western blotting was used to identify the potential signaling pathways that are affected in glioma cell growth by miR-423-5p. Xenograft tumors were examined in vivo for the carcinogenic effects of miR-423-5p in glioma tissues.
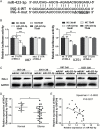
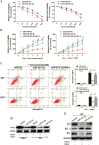
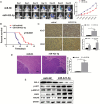
Results: We first reported that miR-423-5p expression was increased in gliomas and was a potential tumor promoter via targeting ING-4. The overexpression of miR-423-5p resulted in upregulation of important signaling molecules such as p-AKT and p-ERK1/2. In clinical samples, miR-423-5p was dysregulated, and a corresponding alteration in ING-4 expression was observed (P = .0207). Furthermore, the overexpression of miR-423-5p strengthened glioma cell proliferation, angiogenesis, and invasion. Finally, miR-423-5p overexpression also strengthened GBM neurosphere formation and rendered glioma cells resistant to temozolomide (TMZ).
Conclusion: This study establishes that miR-423-5p functions as an oncogene in glioma tissues by suppressing ING-4 and suggests that it has therapeutic potential for glioma.
Keywords: ING-4; chemoresistance; glioma; miR-423-5p.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Upregulation of CASC2 sensitized glioma to temozolomide cytotoxicity through autophagy inhibition by sponging miR-193a-5p and regulating mTOR expression.Biomed Pharmacother. 2018 Jan;97:844-850. doi: 10.1016/j.biopha.2017.10.146. Epub 2017 Nov 7. Biomed Pharmacother. 2018. PMID: 29136760
-
MiR-181b-5p modulates chemosensitivity of glioma cells to temozolomide by targeting Bcl-2.Biomed Pharmacother. 2019 Jan;109:2192-2202. doi: 10.1016/j.biopha.2018.11.074. Epub 2018 Nov 27. Biomed Pharmacother. 2019. PMID: 30551476
-
MicroRNA-101 reverses temozolomide resistance by inhibition of GSK3β in glioblastoma.Oncotarget. 2016 Nov 29;7(48):79584-79595. doi: 10.18632/oncotarget.12861. Oncotarget. 2016. PMID: 27792996 Free PMC article.
-
MicroRNAs as the pivotal regulators of Temozolomide resistance in glioblastoma.Mol Brain. 2024 Jul 2;17(1):42. doi: 10.1186/s13041-024-01113-6. Mol Brain. 2024. PMID: 38956588 Free PMC article. Review.
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene.BMC Cancer. 2022 Jun 11;22(1):642. doi: 10.1186/s12885-022-09682-2. BMC Cancer. 2022. PMID: 35690717 Free PMC article. Review.
Cited by
-
Long Non-coding RNA FENDRR Acts as a miR-423-5p Sponge to Suppress the Treg-Mediated Immune Escape of Hepatocellular Carcinoma Cells.Mol Ther Nucleic Acids. 2019 Sep 6;17:516-529. doi: 10.1016/j.omtn.2019.05.027. Epub 2019 Jun 12. Mol Ther Nucleic Acids. 2019. PMID: 31351327 Free PMC article.
-
circ_0003418 Inhibits Tumorigenesis And Cisplatin Chemoresistance Through Wnt/β-Catenin Pathway In Hepatocellular Carcinoma.Onco Targets Ther. 2019 Nov 11;12:9539-9549. doi: 10.2147/OTT.S229507. eCollection 2019. Onco Targets Ther. 2019. PMID: 31807029 Free PMC article.
-
MiR-218-5p targets LHFPL3 to regulate proliferation, migration, and epithelial-mesenchymal transitions of human glioma cells.Biosci Rep. 2019 Mar 1;39(3):BSR20180879. doi: 10.1042/BSR20180879. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30314994 Free PMC article.
-
Oncogenic Role of MicroRNA-30b-5p in Glioblastoma Through Targeting Proline-Rich Transmembrane Protein 2.Oncol Res. 2018 Mar 5;26(2):219-230. doi: 10.3727/096504017X14944585873659. Epub 2017 May 17. Oncol Res. 2018. PMID: 28550683 Free PMC article.
-
Unlocking temozolomide resistance in glioblastoma: the pivotal role of MicroRNAs and in-silico insights.Med Oncol. 2025 Jul 17;42(8):343. doi: 10.1007/s12032-025-02884-1. Med Oncol. 2025. PMID: 40676394 Review.
References
-
- Huse JT, Holland E, DeAngelis LM. Glioblastoma: molecular analysis and clinical implications. Ann Rev Med. 2013;64:59–70. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. New Engl J Med. 2008;359 (5):492–507. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352 (10):987–996. - PubMed
-
- Janga SC, Vallabhaneni S. MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol. 2011;722:59–74. - PubMed
-
- Chen CZ. MicroRNAs as oncogenes and tumor suppressors. New Eng J Med. 2005;353 (17):1768–1771. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

