Restoration of intestinal function in an MPTP model of Parkinson's Disease
- PMID: 27471168
- PMCID: PMC4965866
- DOI: 10.1038/srep30269
Restoration of intestinal function in an MPTP model of Parkinson's Disease
Abstract
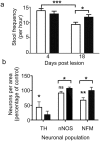
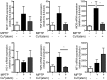
Patients with Parkinson's disease often experience non-motor symptoms including constipation, which manifest prior to the onset of debilitating motor signs. Understanding the causes of these non-motor deficits and developing disease modifying therapeutic strategies has the potential to prevent disease progression. Specific neuronal subpopulations were reduced within the myenteric plexus of mice 21 days after intoxication by the intraperitoneal administration of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) and was associated with a reduction in stool frequency, indicative of intestinal dysfunction. Oral administration of the divalent copper complex, Cu(II)(atsm), which has been shown to be neuroprotective and restore motor performance to MPTP lesioned mice, improved stool frequency and was correlated with restoration of neuronal subpopulations in the myenteric plexus of MPTP lesioned mice. Restoration of intestinal function was associated with reduced enteric glial cell reactivity and reduction of markers of inflammation. Therapeutics that have been shown to be neuroprotective in the central nervous system, such as Cu(II)(atsm), therefore also provide symptom relief and are disease modifying in the intestinal tract, suggesting that there is a common cause of Parkinson's disease pathogenesis in the enteric nervous system and central nervous system.
Conflict of interest statement
Patent protection has previously been sought by the University of Melbourne for the use of bis(- thiosemicarbazones) for treatment of diseases. Professor White and Professor Barnham are co-inventors on this patent application PCT/AU2007/001792, which is the subject of a commercialization contract between the University and Collaborative Medicinal Development. The company has not funded nor contributed to research described in this manuscript.
Figures

Similar articles
-
Gene dysregulation is restored in the Parkinson's disease MPTP neurotoxic mice model upon treatment of the therapeutic drug Cu(II)(atsm).Sci Rep. 2016 Mar 1;6:22398. doi: 10.1038/srep22398. Sci Rep. 2016. PMID: 26928495 Free PMC article.
-
Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents.Neuropharmacology. 2015 Dec;99:448-58. doi: 10.1016/j.neuropharm.2015.08.016. Epub 2015 Aug 14. Neuropharmacology. 2015. PMID: 26282118
-
Neuroprotective effects of madecassoside in early stage of Parkinson's disease induced by MPTP in rats.Fitoterapia. 2013 Oct;90:112-8. doi: 10.1016/j.fitote.2013.07.009. Epub 2013 Jul 20. Fitoterapia. 2013. PMID: 23876367
-
Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson's disease.J Neuroendocrinol. 2020 Jan;32(1):e12782. doi: 10.1111/jne.12782. Epub 2019 Sep 10. J Neuroendocrinol. 2020. PMID: 31430407
-
Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease.Med Sci Monit. 2005 Jan;11(1):RA17-23. Med Sci Monit. 2005. PMID: 15614202 Review.
Cited by
-
Impairment of Nrf2- and Nitrergic-Mediated Gastrointestinal Motility in an MPTP Mouse Model of Parkinson's Disease.Dig Dis Sci. 2019 Dec;64(12):3502-3517. doi: 10.1007/s10620-019-05693-5. Epub 2019 Jun 11. Dig Dis Sci. 2019. PMID: 31187328 Free PMC article.
-
Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson's Disease.Neurochem Res. 2018 Oct;43(10):1986-1999. doi: 10.1007/s11064-018-2620-x. Epub 2018 Aug 31. Neurochem Res. 2018. PMID: 30171422
-
Contractile dysfunction and nitrergic dysregulation in small intestine of a primate model of Parkinson's disease.NPJ Parkinsons Dis. 2019 Jun 10;5:10. doi: 10.1038/s41531-019-0081-9. eCollection 2019. NPJ Parkinsons Dis. 2019. PMID: 31231674 Free PMC article.
-
Gastrointestinal dysmotility in rodent models of Parkinson's disease.Am J Physiol Gastrointest Liver Physiol. 2024 Apr 1;326(4):G345-G359. doi: 10.1152/ajpgi.00225.2023. Epub 2024 Jan 23. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38261717 Free PMC article. Review.
-
Parkinson's Disease and the Gut: Future Perspectives for Early Diagnosis.Front Neurosci. 2020 Jun 17;14:626. doi: 10.3389/fnins.2020.00626. eCollection 2020. Front Neurosci. 2020. PMID: 32625058 Free PMC article. Review.
References
-
- Natale G., Pasquali L., Ruggieri S., Paparelli A. & Fornai F. Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 20, 741–749, doi: 10.1111/j.1365-2982.2008.01162.x (2008). - DOI - PubMed
-
- Abbott R. D. et al.. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–462 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

