Cold acclimation is accompanied by complex responses of glycosylphosphatidylinositol (GPI)-anchored proteins in Arabidopsis
- PMID: 27471282
- PMCID: PMC5014161
- DOI: 10.1093/jxb/erw279
Cold acclimation is accompanied by complex responses of glycosylphosphatidylinositol (GPI)-anchored proteins in Arabidopsis
Abstract
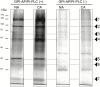
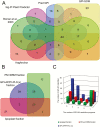
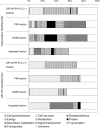
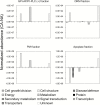
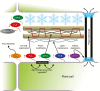
Cold acclimation results in changes of the plasma membrane (PM) composition. The PM is considered to contain specific lipid/protein-enriched microdomains which can be extracted as detergent-resistant plasma membrane (DRM). Previous studies in animal cells have demonstrated that glycosylphosphatidylinositol-anchored proteins (GPI-APs) can be targeted to microdomains and/or the apoplast. However, the functional significance of GPI-APs during cold acclimation in plants is not yet fully understood. In this study, we aimed to investigate the responsiveness of GPI-APs to cold acclimation treatment in Arabidopsis We isolated the PM, DRM, and apoplast fractions separately and, in addition, GPI-AP-enriched fractions were prepared from the PM preparation. Label-free quantitative shotgun proteomics identified a number of GPI-APs (163 proteins). Among them, some GPI-APs such as fasciclin-like arabinogalactan proteins and glycerophosphoryldiester phosphodiesterase-like proteins predominantly increased in PM- and GPI-AP-enriched fractions while the changes of GPI-APs in the DRM and apoplast fractions during cold acclimation were considerably different from those of other fractions. These proteins are thought to be associated with cell wall structure and properties. Therefore, this study demonstrated that each GPI-AP responded to cold acclimation in a different manner, suggesting that these changes during cold acclimation are involved in rearrangement of the extracellular matrix including the cell wall towards acquisition of freezing tolerance.
Keywords: Apoplast; Arabidopsis; GPI-anchored protein; cold acclimation; freezing tolerance; microdomain; plasma membrane; proteomics..
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Plasma membrane proteomic changes of Arabidopsis DRP1E during cold acclimation in association with the enhancement of freezing tolerance.Physiol Plant. 2022 Nov;174(6):e13820. doi: 10.1111/ppl.13820. Physiol Plant. 2022. PMID: 36335535
-
Modification-specific proteomics of plasma membrane proteins: identification and characterization of glycosylphosphatidylinositol-anchored proteins released upon phospholipase D treatment.J Proteome Res. 2006 Apr;5(4):935-43. doi: 10.1021/pr050419u. J Proteome Res. 2006. PMID: 16602701
-
Temporal proteomics of Arabidopsis plasma membrane during cold- and de-acclimation.J Proteomics. 2019 Apr 15;197:71-81. doi: 10.1016/j.jprot.2018.11.008. Epub 2018 Nov 14. J Proteomics. 2019. PMID: 30447334
-
Recent research progress in glycosylphosphatidylinositol-anchored protein biosynthesis, chemical/chemoenzymatic synthesis, and interaction with the cell membrane.Curr Opin Chem Biol. 2024 Feb;78:102421. doi: 10.1016/j.cbpa.2023.102421. Epub 2024 Jan 4. Curr Opin Chem Biol. 2024. PMID: 38181647 Free PMC article. Review.
-
Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport.Biochim Biophys Acta. 2016 Apr;1858(4):632-9. doi: 10.1016/j.bbamem.2015.12.018. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26706096 Review.
Cited by
-
Fasciclin-like arabinogalactan gene family in Nicotiana benthamiana: genome-wide identification, classification and expression in response to pathogens.BMC Plant Biol. 2020 Jul 1;20(1):305. doi: 10.1186/s12870-020-02501-5. BMC Plant Biol. 2020. PMID: 32611364 Free PMC article.
-
Tissue-specific changes in apoplastic proteins and cell wall structure during cold acclimation of winter wheat crowns.J Exp Bot. 2018 Feb 23;69(5):1221-1234. doi: 10.1093/jxb/erx450. J Exp Bot. 2018. PMID: 29373702 Free PMC article.
-
Genome-wide identification and analysis of the FLA gene family in maize (Zea mays L.) and its expression in response to abiotic stresses.BMC Plant Biol. 2025 Jul 30;25(1):982. doi: 10.1186/s12870-025-06981-1. BMC Plant Biol. 2025. PMID: 40739182 Free PMC article.
-
Nitric Oxide Controls Constitutive Freezing Tolerance in Arabidopsis by Attenuating the Levels of Osmoprotectants, Stress-Related Hormones and Anthocyanins.Sci Rep. 2018 Jun 18;8(1):9268. doi: 10.1038/s41598-018-27668-8. Sci Rep. 2018. PMID: 29915353 Free PMC article.
-
Glycosylphosphatidylinositol-Anchored Proteins in Arabidopsis and One of Their Common Roles in Signaling Transduction.Front Plant Sci. 2019 Aug 29;10:1022. doi: 10.3389/fpls.2019.01022. eCollection 2019. Front Plant Sci. 2019. PMID: 31555307 Free PMC article. Review.
References
-
- Alexandersson E, Saalbach G, Larsson C, Kjellbom P. 2004. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant and Cell Physiology 45, 1543–1556. - PubMed
-
- Baldwin L, Domon J-M, Klimek JF, Fournet F, Sellier H, Gillet F, Pelloux J, Lejeune-Hénaut I, Carpita NC, Rayon C. 2014. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104, 37–47. - PubMed
-
- Bevan M, Bancroft I, Bent E, et al. 1998. Analysis of 1.9Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391, 485–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

