Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy
- PMID: 27471616
- PMCID: PMC4938314
- DOI: 10.1080/2162402X.2016.1149673
Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy
Abstract
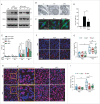
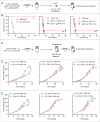
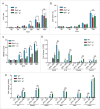
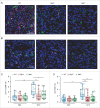
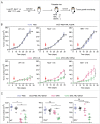
Chemotherapy can reinstate anticancer immunosurveillance through inducing tumor immunogenic cell death (ICD). Here, we show that anthracyclines and oxaliplatin can trigger necroptosis in murine cancer cell lines expressing receptor-interacting serine-threonine kinase 3 (RIP3) and mixed lineage kinase domain-like (MLKL). Necroptotic cells featured biochemical hallmarks of ICD and stimulated anticancer immune responses in vivo. Chemotherapy normally killed Rip3 (-/-) and Mlkl (-/-) tumor cells and normally induced caspase-3 activation in such cells, yet was unable to reduce their growth in vivo. RIP3 or MLKL deficiency abolished the capacity of dying cancer cells to elicit an immune response. This could be attributed to reduced release of ATP and high mobility group box 1 (HMGB1) by RIP3 and MLKL-deficient cells. Measures designed to compensate for deficient ATP and HMGB1 signaling restored the chemotherapeutic response of Rip3 (-/-) and Mlkl (-/-) cancers. Altogether, these results suggest that RIP3 and MLKL can contribute to ICD signaling and tumor immunogenicity.
Keywords: ATP; HMGB1; Necroptosis; Tumor immunogenicity; chemotherapy; cytotoxic T cells; dendritic cells.
Figures


References
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:21219185; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101324 - DOI - PubMed
-
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161:205-14; PMID:25860605; http://dx.doi.org/ 10.1016/j.cell.2015.03.030 - DOI - PMC - PubMed
-
- Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P et al.. Classification of current anticancer immunotherapies. Oncotarget 2014; 5:12472-508; PMID:25537519; http://dx.doi.org/ 10.18632/oncotarget.2998 - DOI - PMC - PubMed
-
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:23890065; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 - DOI - PubMed
-
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/ 10.1200/JCO.2009.23.7370 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
