Update on use of aldesleukin for treatment of high-risk metastatic melanoma
- PMID: 27471714
- PMCID: PMC4918260
- DOI: 10.2147/ITT.S61590
Update on use of aldesleukin for treatment of high-risk metastatic melanoma
Abstract
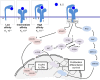
High-dose interleukin-2 has been used for the treatment of metastatic melanoma since 1998 based on data proving durable complete responses in up to 10% of treated patients. The immunomodulatory effects of this critical cytokine have been instrumental in the development of immunotherapy for melanoma and other cancers. However, with the advent of new therapies, its use as a front-line agent has come into question. Nonetheless, there is still a role for interleukin-2 as monotherapy, as well as in combination with other agents and in clinical trials. In this article, we review preclinical and clinical data regarding interleukin-2, its pharmacology and mechanism of action, its toxicity profile, and its use in ongoing and planned clinical trials. We also explore the future of this agent within the treatment landscape for melanoma.
Keywords: aldesleukin; immunotherapy; melanoma.
Figures

References
-
- Carbone PP, Costello W. Eastern Cooperative Oncology Group studies with DTIC (NSC-45388) Cancer Treat Rep. 1976;60(2):193–198. - PubMed
-
- Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) Eur J Cancer. 2011;47(10):1476–1483. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. - PubMed
-
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

