Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice
- PMID: 27472084
- PMCID: PMC5026820
- DOI: 10.1080/15384101.2016.1201255
Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice
Abstract
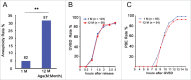
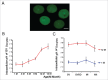
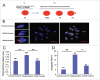
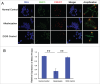
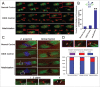
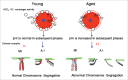
Increases in the aneuploidy rate caused by the deterioration of cohesion with increasing maternal age have been well documented. However, the molecular mechanism for the loss of cohesion in aged oocytes remains unknown. In this study, we found that intracellular pH (pHi) was elevated in aged oocytes, which might disturb the structure of the cohesin ring to induce aneuploidy. We observed for the first time that full-grown germinal vesicle (GV) oocytes displayed an increase in pHi with advancing age in CD1 mice. Furthermore, during the in vitro oocyte maturation process, the pHi was maintained at a high level, up to ∼7.6, in 12-month-old mice. Normal pHi is necessary to maintain protein localization and function. Thus, we put forward a hypothesis that the elevated oocyte pHi might be related to the loss of cohesion and the increased aneuploidy in aged mice. Through the in vitro alkalinization treatment of young oocytes, we observed that the increased pHi caused an increase in the aneuploidy rate and the sister inter-kinetochore (iKT) distance associated with the strength of cohesion and caused a decline in the cohesin subunit SMC3 protein level. Young oocytes with elevated pHi exhibited substantially the increase in chromosome misalignment.
Keywords: aging; alkalinization; chromosome aneuploidy; cohesion; oocytes; pHi.
Figures

References
-
- Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 2008; 316:397-407; PMID:18342300; http://dx.doi.org/10.1016/j.ydbio.2008.01.048 - DOI - PMC - PubMed
-
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 2012; 13:493-504; PMID:22705668; http://dx.doi.org/10.1038/nrg3245 - DOI - PMC - PubMed
-
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010; 20:1522-8; PMID:20817534; http://dx.doi.org/10.1016/j.cub.2010.06.069 - DOI - PMC - PubMed
-
- Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod 2012; 86:1-7; PMID:21957193; http://dx.doi.org/10.1095/biolreprod.111.094367 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
