iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes
- PMID: 27472162
- PMCID: PMC5687559
- DOI: 10.1016/j.biomaterials.2016.07.023
iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes
Abstract
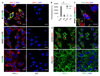
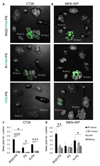
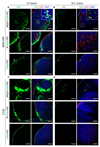
Polymersomes are versatile nanoscale vesicles that can be used for cytoplasmic delivery of payloads. Recently, we demonstrated that pH-sensitive polymersomes exhibit an intrinsic selectivity towards intraperitoneal tumor lesions. A tumor homing peptide, iRGD, harbors a cryptic C-end Rule (CendR) motif that is responsible for neuropilin-1 (NRP-1) binding and for triggering extravasation and tumor penetration of the peptide. iRGD functionalization increases tumor selectivity and therapeutic efficacy of systemic drug-loaded nanoparticles in many tumor models. Here we studied whether intraperitoneally administered paclitaxel-loaded iRGD-polymersomes show improved efficacy in the treatment of peritoneal carcinomatosis. First, we demonstrated that the pH-sensitive polymersomes functionalized with RPARPAR (a prototypic CendR peptide) or iRGD internalize in the cells that express NRP-1, and that internalized polymersomes release their cargo inside the cytosol. CendR-targeted polymersomes loaded with paclitaxel were more cytotoxic on NRP-1-positive cells than on NRP-1-negative cells. In mice bearing peritoneal tumors of gastric (MKN-45P) or colon (CT26) origin, intraperitoneally administered RPARPAR and iRGD-polymersomes showed higher tumor-selective accumulation and penetration than untargeted polymersomes. Finally, iRGD-polymersomes loaded with paclitaxel showed improved efficacy in peritoneal tumor growth inhibition and in suppression of local dissemination compared to the pristine paclitaxel-polymersomes or Abraxane. Our study demonstrates that iRGD-functionalization improves efficacy of paclitaxel-polymersomes for intraperitoneal treatment of peritoneal carcinomatosis.
Keywords: NRP-1; Paclitaxel; Peritoneal carcinomatosis; Polymersomes; Tumor penetrating peptides; iRGD.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

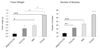
Similar articles
-
Paclitaxel-Loaded Polymersomes for Enhanced Intraperitoneal Chemotherapy.Mol Cancer Ther. 2016 Apr;15(4):670-9. doi: 10.1158/1535-7163.MCT-15-0713-T. Epub 2016 Feb 15. Mol Cancer Ther. 2016. PMID: 26880267 Free PMC article.
-
Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment.Int J Nanomedicine. 2019 Nov 1;14:8543-8560. doi: 10.2147/IJN.S219820. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31802868 Free PMC article.
-
Targeted Modification of the Cationic Anticancer Peptide HPRP-A1 with iRGD To Improve Specificity, Penetration, and Tumor-Tissue Accumulation.Mol Pharm. 2019 Feb 4;16(2):561-572. doi: 10.1021/acs.molpharmaceut.8b00854. Epub 2019 Jan 14. Mol Pharm. 2019. PMID: 30592418
-
iRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery.Polymers (Basel). 2020 Aug 24;12(9):1906. doi: 10.3390/polym12091906. Polymers (Basel). 2020. PMID: 32847045 Free PMC article. Review.
-
Recent advances in the tumor-penetrating peptide internalizing RGD for cancer treatment and diagnosis.Drug Dev Res. 2023 Jun;84(4):654-670. doi: 10.1002/ddr.22056. Epub 2023 Apr 5. Drug Dev Res. 2023. PMID: 36959702 Review.
Cited by
-
Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics.Pharmaceutics. 2021 Aug 30;13(9):1365. doi: 10.3390/pharmaceutics13091365. Pharmaceutics. 2021. PMID: 34575441 Free PMC article. Review.
-
ROS-responsive dimeric prodrug-based nanomedicine targeted therapy for gastric cancer.Drug Deliv. 2021 Dec;28(1):1204-1213. doi: 10.1080/10717544.2021.1937380. Drug Deliv. 2021. PMID: 34142633 Free PMC article.
-
Maximized nanodrug-loaded mesenchymal stem cells by a dual drug-loaded mode for the systemic treatment of metastatic lung cancer.Drug Deliv. 2017 Nov;24(1):1372-1383. doi: 10.1080/10717544.2017.1375580. Drug Deliv. 2017. PMID: 28920712 Free PMC article.
-
Bi-Functional Peptides as a New Therapeutic Tool for Hepatocellular Carcinoma.Pharmaceutics. 2021 Oct 6;13(10):1631. doi: 10.3390/pharmaceutics13101631. Pharmaceutics. 2021. PMID: 34683924 Free PMC article.
-
In vivo cation exchange in quantum dots for tumor-specific imaging.Nat Commun. 2017 Aug 24;8(1):343. doi: 10.1038/s41467-017-00153-y. Nat Commun. 2017. PMID: 28839238 Free PMC article.
References
-
- Intraperitoneal Cancer Therapy: Principles and Practice. CRC Press Book; (n.d.). https://www.crcpress.com/Intraperitoneal-Cancer-Therapy-Principles-and-P... (accessed 04.24.16).
-
- Eskander RN. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in epithelial ovarian cancer: state of the art. World J Obstet Gynecol. 2013;2:94. doi: 10.5317/wjog.v2.i4.94. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

