Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports
- PMID: 27472273
- PMCID: PMC4966895
- DOI: 10.1371/journal.pone.0160221
Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports
Abstract
Background: Three checkpoint inhibitor drugs have been approved by the US Food and Drug Administration for use in specific types of cancers. While the results are promising, severe immunotherapy-related adverse events (irAEs) have been reported.
Objectives: To conduct a systematic review of case reports describing the occurrence of irAEs in patients with cancer following checkpoint blockade therapy, primarily to identify potentially unrecognized or unusual clinical findings and toxicity.
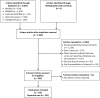
Data sources: We searched Medline, EMBASE, Web of Science, PubMed ePubs, and Cochrane CENTRAL with no restriction through August 2015.
Study selection: Studies reporting cases of cancer develop irAEs following treatment with anti CTLA-4 (ipilimumab) or anti PD-1 (nivolumab or pembrolizumab) antibodies were included.
Data extraction: We extracted data on patient characteristics, irAEs characteristics, how irAEs were managed, and their outcomes.
Data synthesis: 191 publications met inclusion criteria, reporting on 251 cases. Most patients had metastatic melanoma (95.6%), and the majority were treated with ipilimumab (93.2%). Autoimmune colitis, hepatitis, endocrinopathies, and cutaneous irAEs were the most frequently reported irAEs in ipilimumab treated patients. A broad spectrum of toxicities were reported for almost every body system. Moreover, well-defined diseases such as sarcoidosis, polyarthritis, polymyalgia rheumatica/arteritis, lupus, celiac disease, dermatomyositis, and Vogt-Koyanagi-like syndrome were reported. The most frequent irAEs reported with anti-PD1 agents were dermatitis for pembrolizumab, and thyroid disease and pneumonitis for nivolumab. Complete resolution of adverse events occurred in most cases. However, persistent irAEs and death were reported, mainly in patients treated with ipilimumab.
Limitations: Our study is limited by information available in the original reports.
Conclusions: Evidence from case reports shows that cancer patients develop irAEs following checkpoint blockade therapy, and can occasionally develop clearly defined autoimmune systemic diseases. While discontinuation of therapy and/or treatment can result in resolution of irAEs, long-term sequelae and death have been reported.
Conflict of interest statement
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous