Antibiotic perturbation of the preterm infant gut microbiome and resistome
- PMID: 27472377
- PMCID: PMC5154371
- DOI: 10.1080/19490976.2016.1218584
Antibiotic perturbation of the preterm infant gut microbiome and resistome
Abstract
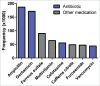
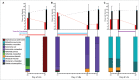
The gut microbiota plays important roles in nutrient absorption, immune system development, and pathogen colonization resistance. Perturbations early in life may be detrimental to host health in the short and the long-term. Antibiotics are among the many factors that influence the development of the microbiota. Because antibiotics are heavily administered during the first critical years of gut microbiota development, it is important to understand the effects of these interventions. Infants, particularly those born prematurely, represent an interesting population because they receive early and often extensive antibiotic therapy in the first months after birth. Gibson et al. recently demonstrated that antibiotic therapy in preterm infants can dramatically affect the gut microbiome. While meropenem, ticarcillin-clavulanate, and cefotaxime treatments were associated with decreased species richness, gentamicin and vancomycin had variable effects on species richness. Interestingly, the direction of species richness response could be predicted based on the abundance of 2 species and 2 genes in the microbiome prior to gentamicin or vancomycin treatment. Nonetheless, all antibiotic treatments enriched the presence of resistance genes and multidrug resistant organisms. Treatment with different antibiotics further resulted in unique population shifts of abundant organisms and selection for different sets of resistance genes. In this addendum, we provide an extended discussion of these recent findings, and outline important future directions for elucidating the interplay between antibiotics and preterm infant gut microbiota development.
Keywords: antibiotic resistance; antibiotics; gut microbiota; preterm infants.
Figures
Erratum for
- doi: 10.1038/nmicrobiol.2016.24
References
-
- Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med 2016; 22:713-22; PMID:27387886; http://dx.doi.org/ 10.1038/nm.4142 - DOI - PubMed
-
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014; 6:237ra65; PMID:24848255; http://dx.doi.org/ 10.1126/scitranslmed.3008599 - DOI - PMC - PubMed
-
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al.. Human gut microbiome viewed across age and geography. Nature 2012; 486:222-7; PMID:22699611; http://dx.doi.org/ 10.1038/nature11053 - DOI - PMC - PubMed
-
- La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE Jr., Shaikh N, Linneman LA, et al.. Patterned progression of bacterial populations in the premature infant gut. Pro Natl Acad Sci U S A 2014; 111:12522-7; PMID:25114261; http://dx.doi.org/ 10.1073/pnas.1409497111 - DOI - PMC - PubMed
-
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al.. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015; 17:690-703; PMID:25974306; http://dx.doi.org/ 10.1016/j.chom.2015.04.004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
