Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer
- PMID: 27473061
- PMCID: PMC4966732
- DOI: 10.1186/s13058-016-0737-x
Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer
Abstract
Background: Tumor-infiltrating lymphocyte (TIL) count in breast cancer carries prognostic information and represents a potential predictive marker for emerging immunotherapies. However, the distribution of the lymphocyte subpopulations is not well defined. The goals of this study were to examine intratumor heterogeneity in TIL subpopulation counts in different fields of view (FOV) within each section, in different sections from the same biopsy, and between biopsies from different regions of the same cancer using quantitative immunofluorescence (QIF).
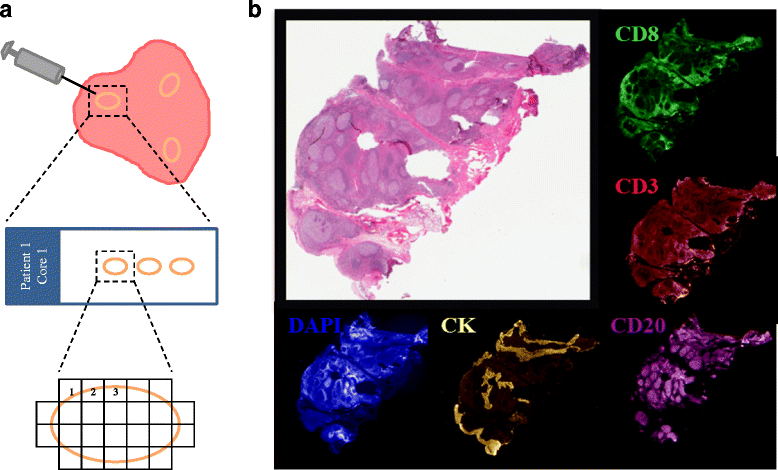
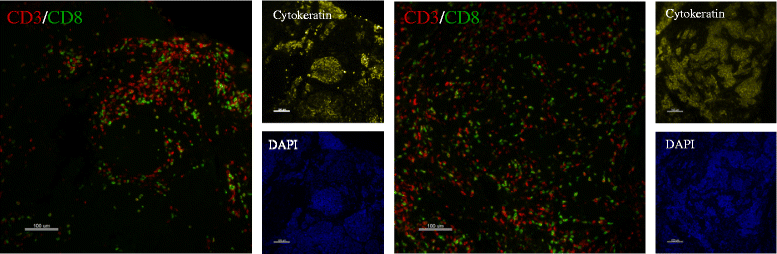
Methods: We used multiplexed QIF to quantify cytokeratin-positive epithelial cells, and CD3-positive, CD8-positive and CD20-positive lymphocytes in tissue sections from multiple biopsies obtained from different areas of 31 surgically resected primary breast carcinomas (93 samples total). Log2-transformed QIF scores or concordance and variance component analyses with linear mixed-effects models were used. Cohen's kappa index [k] of high versus low scores, defined as above and below the median, was used to measure sample similarity between areas.
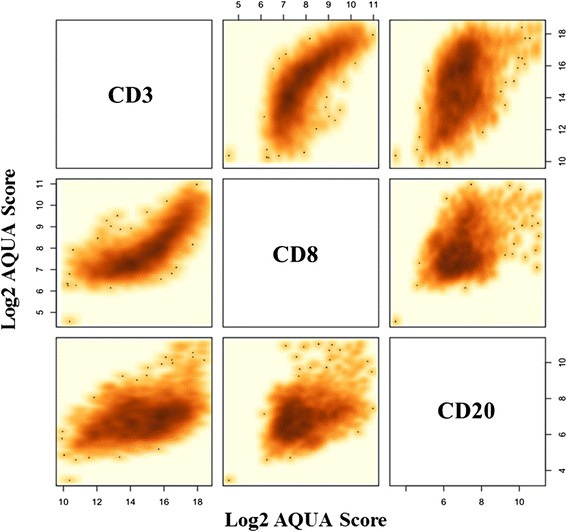
Results: We found a strong positive correlation between CD3 and CD8 levels across all patients (Pearson correlation coefficient [CC] = 0.827). CD3 and CD8 showed a weaker but significant association with CD20 (CC = 0.446 and 0.363, respectively). For each marker, the variation between different FOVs in the same section was higher than the variation between sections or between biopsies of the same cancer. The intraclass correlation coefficients (ICC) were 0.411 for CD3, 0.324 for CD8, and 0.252 for CD20. In component analysis, 66-69 % of the variance was attributable to differences between FOVs in the same section and 30-33 % was due to differences between biopsies from different areas of the same cancer. Section to section differences were negligible. Concordance for low versus high marker status assignment in single biopsies compared to all three biopsies combined yielded k = 0.705 for CD3, k = 0.655 for CD8, and k = 0.603 for CD20.
Conclusions: T and B lymphocytes show more heterogeneity across the dimensions of a single section than between different sections or regions of a given breast tumor. This observation suggests that the average lymphocyte score from a single biopsy of a tumor is reasonably representative of the whole cancer.
Keywords: B cells; Breast cancer; Immunofluorescence; Stroma; T cells; Tumor microenvironment.
Figures


References
-
- Salgado R, Denkert C, Campbell C, Savas P, Nucifero P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1(4):448–54. doi: 10.1001/jamaoncol.2015.0830. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

