Integrin αDβ2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS)
- PMID: 27473068
- PMCID: PMC4967320
- DOI: 10.1186/s12936-016-1447-7
Integrin αDβ2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS)
Abstract
Background: Malaria-associated acute respiratory distress syndrome (MA-ARDS) is a potentially lethal complication of clinical malaria. Acute lung injury in MA-ARDS shares features with ARDS triggered by other causes, including alveolar inflammation and increased alveolar-capillary permeability, leading to leak of protein-rich pulmonary oedema fluid. Mechanisms and physiologic alterations in MA-ARDS can be examined in murine models of this syndrome. Integrin αDβ2 is a member of the leukocyte, or β2 (CD18), sub-family of integrins, and emerging observations indicate that it has important activities in leukocyte adhesion, accumulation and signalling. The goal was to perform analysis of the lungs of mice wild type C57Bl/6 (a D (+/+) ) and Knockout C57Bl/6 (a D (-/-) ) with malaria-associated acute lung injury to better determine the relevancy of the murine models and investigate the mechanism of disease.
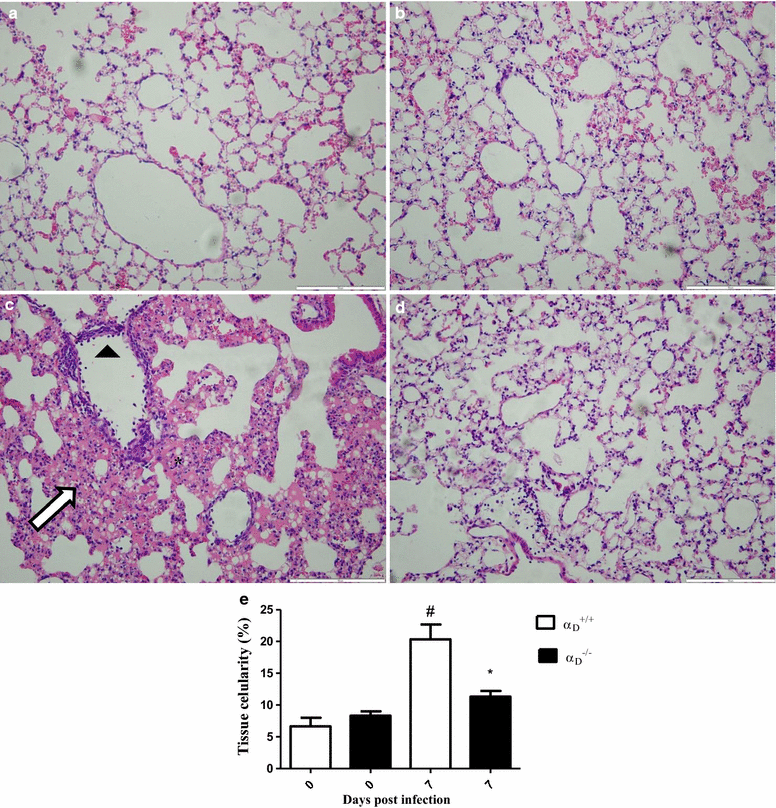
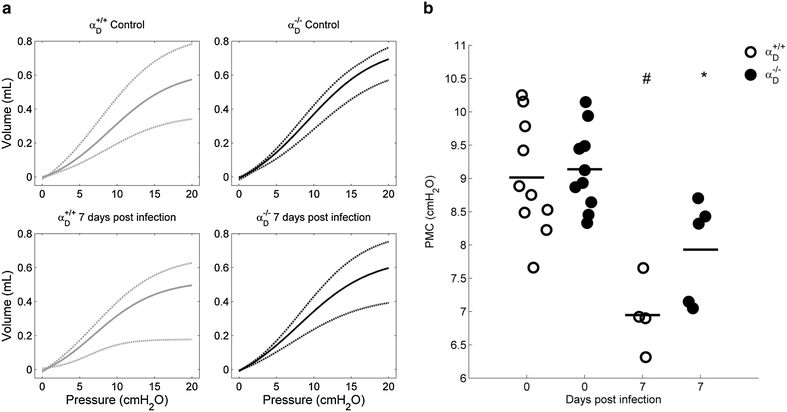
Methods: C57BL/6 wild type (a D (+/+) ) and deficient for CD11d sub-unit (a D (-/-) ) mice were monitored after infection with 10(5) Plasmodium berghei ANKA. CD11d subunit expression RNA was measured by real-time polymerase chain reaction, vascular barrier integrity by Evans blue dye (EBD) exclusion and cytokines by ELISA. Protein and leukocytes were measured in bronchoalveolar lavage fluid (BALF) samples. Tissue cellularity was measured by the point-counting technique, F4/80 and VCAM-1 expression by immunohistochemistry. Respiratory function was analysed by non-invasive BUXCO and mechanical ventilation.
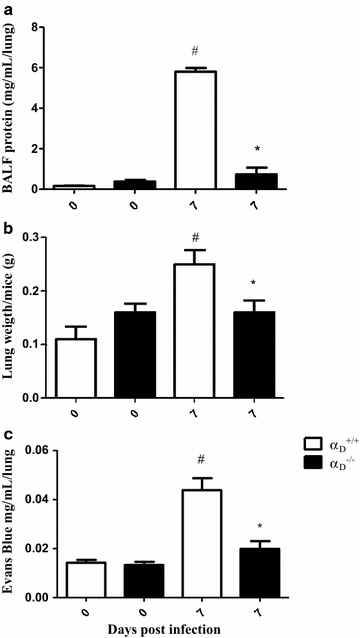
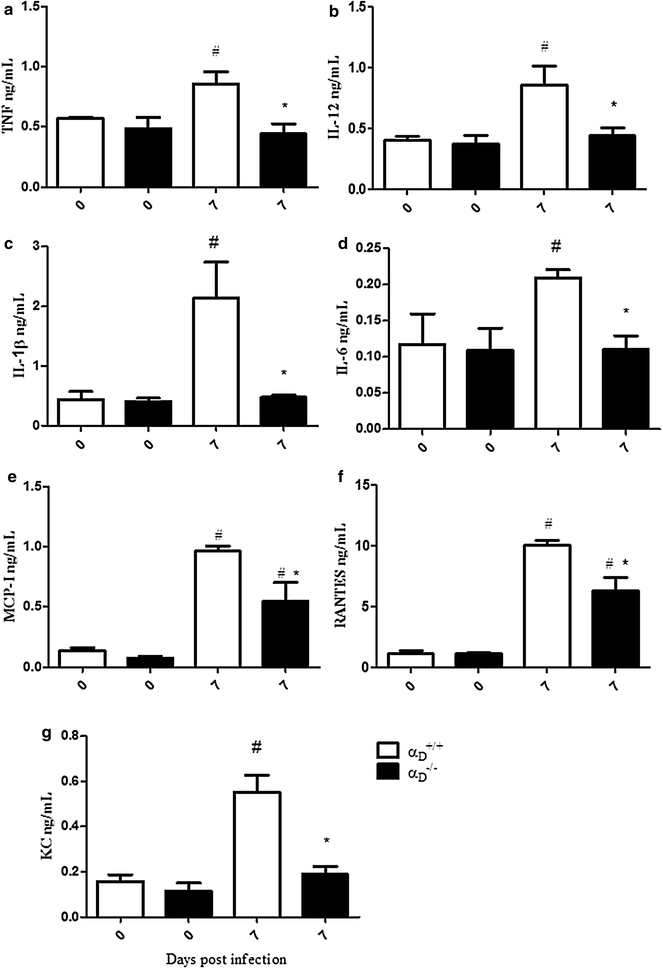
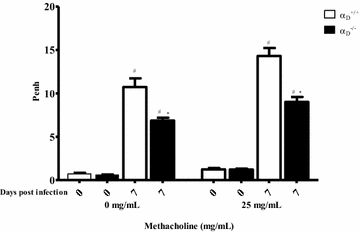
Results: Alveolar inflammation, vascular and interstitial accumulation of monocytes and macrophages, and disrupted alveolar-capillary barrier function with exudation of protein-rich pulmonary oedema fluid were present in P. berghei-infected wild type mice and were improved in αDβ2-deficient animals. Key pro-inflammatory cytokines were also decreased in lung tissue from α D (-/-) mice, providing a mechanistic explanation for reduced alveolar-capillary inflammation and leak.
Conclusions: The results indicate that αDβ2 is an important inflammatory effector molecule in P. berghei-induced MA-ARDS, and that leukocyte integrins regulate critical inflammatory and pathophysiologic events in this model of complicated malaria. Genetic deletion of integrin subunit αD in mice, leading to deficiency of integrin αDβ2, alters lung inflammation and acute lung injury in a mouse model of MA-ARDS caused by P. berghei.
Keywords: Acute lung injury; Acute respiratory distress syndrome; Inflammation; Integrin αDβ2; Malaria.
Figures


References
-
- WHO . World malaria report 2015. Geneva: World Health Organization; 2015. pp. 8–21.
-
- Taylor BM, Kolbasa KP, Chin JE, Richards IM, Fleming WE, Griffin RL, et al. Roles of adhesion molecules ICAM-1 and α4 integrin in antigen-induced changes in microvascular permeability associated with lung inflammation in sensitized brown Norway rats. Am J Respir Cell Mol Biol. 1997;17:757–766. doi: 10.1165/ajrcmb.17.6.2697. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

