Cognitive behavioral therapy program for cannabis use cessation in first-episode psychosis patients: study protocol for a randomized controlled trial
- PMID: 27473688
- PMCID: PMC4966873
- DOI: 10.1186/s13063-016-1507-x
Cognitive behavioral therapy program for cannabis use cessation in first-episode psychosis patients: study protocol for a randomized controlled trial
Abstract
Background: The high rate of cannabis use among patients with first-episode psychosis (FEP), as well as the associated negative impact on illness course and treatment outcomes, underlines the need for effective interventions in these populations. However, to date, there have been few clinical treatment trials (of pharmacological or psychological interventions) that have specifically focused on addressing comorbid cannabis use among these patients. The aim of this paper is to describe the design of a study protocol for a randomized controlled trial in which the objective is to assess the efficacy of a specific cognitive behavioral therapy program for cannabis cessation in patients with FEP compared to standard treatment (psychoeducation).
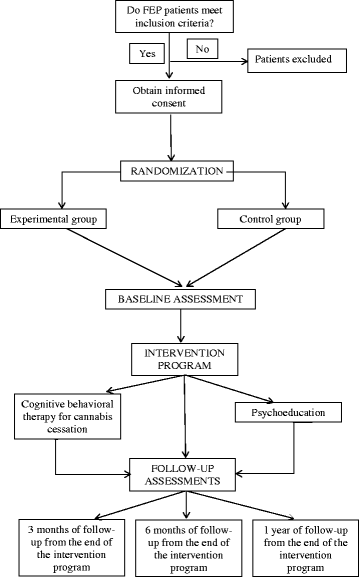
Methods/design: This is a single-blind randomized study with 1 year of follow-up. Patients are to be randomly assigned to one of two treatments: (1) specific cognitive behavioral therapy for cannabis cessation composed of 1-hour sessions once a week for 16 weeks, in addition to pharmacological treatment scheduled by the psychiatrist, or (2) a control group (psychoeducation + pharmacological treatment) following the same format as the experimental group. Participants in both groups will be evaluated at baseline (pre-treatment), at 16 weeks (post-treatment), and at 3 and 6 months and 1 year of follow-up. The primary outcome will be that patients in the experimental group will have greater cannabis cessation than patients in the control group at post-treatment. The secondary outcome will be that the experimental group will have better clinical and functional outcomes than the control group.
Discussion: This study provides the description of a clinical trial design based on specific cognitive behavioral therapy for cannabis cessation in FEP patients, aiming to improve clinical and functional outcome, as well as tackling the addictive disorder.
Trial registration: NCT02319746 ClinicalTrials.gov Identifier. ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 15 December 2014.
Keywords: Cannabis; First-episode psychosis; Psychological treatment.
References
-
- Baeza I, Graell M, Moreno D, Castro-Fornieles J, Parellada M, González-Pinto A, Payá B, Soutullo C, de la Serna E, Arango C. Cannabis use in children and adolescents with first episode psychosis: influence on psychopathology and short-term outcome (CAFEPS study) Schizophr Res. 2009;113:129–37. doi: 10.1016/j.schres.2009.04.005. - DOI - PubMed
-
- Bagot KS, Milin R, Kaminer Y. Adolescent initiation of cannabis use and early-onset psychosis, substance abuse. 2015. 16:0. [Epub ahead of print]. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical