Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin α1β2 in Newly Formed Tertiary Lymphoid Structures
- PMID: 27474071
- PMCID: PMC4991245
- DOI: 10.4049/jimmunol.1500686
Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin α1β2 in Newly Formed Tertiary Lymphoid Structures
Abstract
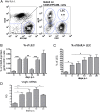
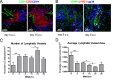
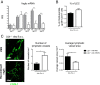
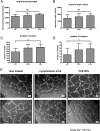
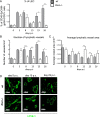
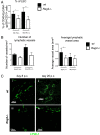
Lymphangiogenesis associated with tertiary lymphoid structure (TLS) has been reported in numerous studies. However, the kinetics and dynamic changes occurring to the lymphatic vascular network during TLS development have not been studied. Using a viral-induced, resolving model of TLS formation in the salivary glands of adult mice we demonstrate that the expansion of the lymphatic vascular network is tightly regulated. Lymphatic vessel expansion occurs in two distinct phases. The first wave of expansion is dependent on IL-7. The second phase, responsible for leukocyte exit from the glands, is regulated by lymphotoxin (LT)βR signaling. These findings, while highlighting the tight regulation of the lymphatic response to inflammation, suggest that targeting the LTα1β2/LTβR pathway in TLS-associated pathologies might impair a natural proresolving mechanism for lymphocyte exit from the tissues and account for the failure of therapeutic strategies that target these molecules in diseases such as rheumatoid arthritis.
Copyright © 2016 The Authors.
Figures


References
-
- Liao S., Ruddle N. H. 2006. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 177: 3369–3379. - PubMed
-
- Kim H., Kataru R. P., Koh G. Y. 2012. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 33: 350–356. - PubMed
-
- Kataru R. P., Jung K., Jang C., Yang H., Schwendener R. A., Baik J. E., Han S. H., Alitalo K., Koh G. Y. 2009. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113: 5650–5659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

