Antibodies That Efficiently Form Hexamers upon Antigen Binding Can Induce Complement-Dependent Cytotoxicity under Complement-Limiting Conditions
- PMID: 27474078
- PMCID: PMC4991250
- DOI: 10.4049/jimmunol.1600648
Antibodies That Efficiently Form Hexamers upon Antigen Binding Can Induce Complement-Dependent Cytotoxicity under Complement-Limiting Conditions
Abstract
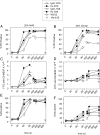
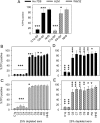
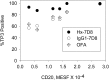
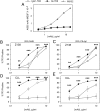
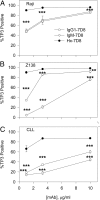
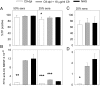
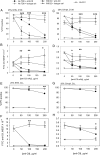
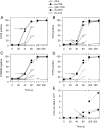
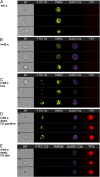
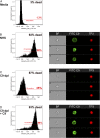
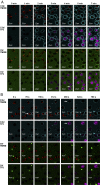
Recently, we demonstrated that IgG Abs can organize into ordered hexamers after binding their cognate Ags expressed on cell surfaces. This process is dependent on Fc:Fc interactions, which promote C1q binding, the first step in classical pathway complement activation. We went on to engineer point mutations that stimulated IgG hexamer formation and complement-dependent cytotoxicity (CDC). The hexamer formation-enhanced (HexaBody) CD20 and CD38 mAbs support faster, more robust CDC than their wild-type counterparts. To further investigate the CDC potential of these mAbs, we used flow cytometry, high-resolution digital imaging, and four-color confocal microscopy to examine their activity against B cell lines and primary chronic lymphocytic leukemia cells in sera depleted of single complement components. We also examined the CDC activity of alemtuzumab (anti-CD52) and mAb W6/32 (anti-HLA), which bind at high density to cells and promote substantial complement activation. Although we observed little CDC for mAb-opsonized cells reacted with sera depleted of early complement components, we were surprised to discover that the Hexabody mAbs, as well as ALM and W6/32, were all quite effective at promoting CDC in sera depleted of individual complement components C6 to C9. However, neutralization studies conducted with an anti-C9 mAb verified that C9 is required for CDC activity against cell lines. These highly effective complement-activating mAbs efficiently focus activated complement components on the cell, including C3b and C9, and promote CDC with a very low threshold of MAC binding, thus providing additional insight into their enhanced efficacy in promoting CDC.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX.J Immunol. 2009 Jul 1;183(1):749-58. doi: 10.4049/jimmunol.0900632. Epub 2009 Jun 17. J Immunol. 2009. PMID: 19535640
-
Combination of two anti-CD5 monoclonal antibodies synergistically induces complement-dependent cytotoxicity of chronic lymphocytic leukaemia cells.Br J Haematol. 2013 Oct;163(2):182-93. doi: 10.1111/bjh.12503. Epub 2013 Aug 8. Br J Haematol. 2013. PMID: 23927424
-
Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia.J Immunol. 2004 Mar 1;172(5):3280-8. doi: 10.4049/jimmunol.172.5.3280. J Immunol. 2004. PMID: 14978136
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
Cytotoxic mechanisms of immunotherapy: Harnessing complement in the action of anti-tumor monoclonal antibodies.Semin Immunol. 2016 Jun;28(3):309-16. doi: 10.1016/j.smim.2016.03.003. Epub 2016 Mar 19. Semin Immunol. 2016. PMID: 27009480 Review.
Cited by
-
The Role of Alpha 2 Macroglobulin in IgG-Aggregation and Chronic Activation of the Complement System in Patients With Chronic Lymphocytic Leukemia.Front Immunol. 2021 Feb 11;11:603569. doi: 10.3389/fimmu.2020.603569. eCollection 2020. Front Immunol. 2021. PMID: 33643290 Free PMC article.
-
The contribution of the alternative pathway in complement activation on cell surfaces depends on the strength of classical pathway initiation.Clin Transl Immunology. 2023 Jan 27;12(1):e1436. doi: 10.1002/cti2.1436. eCollection 2023. Clin Transl Immunology. 2023. PMID: 36721662 Free PMC article.
-
Internalization of HIV-1 by Phagocytes Is Increased When Virions Are Opsonized with Multimeric Antibody in the Presence of Complement.J Virol. 2022 Jan 26;96(2):e0168921. doi: 10.1128/JVI.01689-21. Epub 2021 Nov 3. J Virol. 2022. PMID: 34730392 Free PMC article.
-
Multiple sclerosis plasma IgG aggregates induce complement-dependent neuronal apoptosis.Cell Death Dis. 2023 Apr 8;14(4):254. doi: 10.1038/s41419-023-05783-3. Cell Death Dis. 2023. PMID: 37031195 Free PMC article.
-
Stellabody: A novel hexamer-promoting mutation for improved IgG potency.Immunol Rev. 2024 Nov;328(1):438-455. doi: 10.1111/imr.13400. Epub 2024 Oct 4. Immunol Rev. 2024. PMID: 39364646 Free PMC article. Review.
References
-
- Glennie M. J., French R. R., Cragg M. S., Taylor R. P. 2007. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 44: 3823–3837. - PubMed
-
- Melis J. P. M., Strumane K., Ruuls S. R., Beurskens F. J., Schuurman J., Parren P. W. H. I. 2015. Complement in therapy and disease: regulating the complement system with antibody-based therapeutics. Mol. Immunol. 67(2 Pt A): 117–130. - PubMed
-
- Golay J., Introna M. 2012. Mechanism of action of therapeutic monoclonal antibodies: promises and pitfalls of in vitro and in vivo assays. Arch. Biochem. Biophys. 526: 146–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

