Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells
- PMID: 27474166
- PMCID: PMC5166434
- DOI: 10.1189/jlb.1HI1115-511R
Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells
Abstract
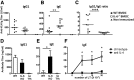
Follicular helper T cells (Tfh) support high-affinity Ab production by germinal center B cells through both membrane interactions and secretion of IL-4 and -21, two major cytokines implicated in B-cell survival and Ab class switch. Tfh-2 cells recently emerged in humans as a strong IL-4 producer Tfh cell subset implicated in both autoimmune and allergic diseases. Although the molecular mechanisms governing Tfh cell differentiation from naive T cells have been widely described, much less is known about the regulation of cytokine secretion by mouse Tfh-2 cells. The purpose of our study was to evaluate the role of dendritic cell-derived IL-6 in fine-tuning cytokine secretion by Tfh cells. Our results demonstrate that priming of Th cells by IL-6-deficient antigen-presenting dendritic cells preferentially leads to accumulation of a subset of Tfh cells characterized by high expression of GATA3 and IL-4, associated with reduced production of IL-21. STAT3-deficient Tfh cells also overexpress GATA3, suggesting that early IL-6/STAT3 signaling during Tfh cell development inhibits the expression of a set of genes associated with the Th2 differentiation program. Overall, our data indicate that IL-6/STAT3 signaling restrains the expression of Th2-like genes in Tfh cells, thus contributing to the control of IgE secretion in vivo.
Keywords: STAT3; Tfh-2; humoral response.
© Society for Leukocyte Biology.
Figures





Comment in
-
Editorial: Masters of fate: the APC cytokine milieu as a key regulator of distinct Tfh cell subsets.J Leukoc Biol. 2017 Jan;101(1):1-3. doi: 10.1189/jlb.1CE0816-351R. J Leukoc Biol. 2017. PMID: 28049141 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

