Inositol hexakisphosphate kinase 1 (IP6K1) activity is required for cytoplasmic dynein-driven transport
- PMID: 27474409
- PMCID: PMC5095903
- DOI: 10.1042/BCJ20160610
Inositol hexakisphosphate kinase 1 (IP6K1) activity is required for cytoplasmic dynein-driven transport
Abstract
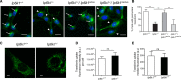
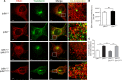
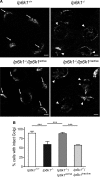
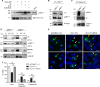
Inositol pyrophosphates, such as diphosphoinositol pentakisphosphate (IP7), are conserved eukaryotic signaling molecules that possess pyrophosphate and monophosphate moieties. Generated predominantly by inositol hexakisphosphate kinases (IP6Ks), inositol pyrophosphates can modulate protein function by posttranslational serine pyrophosphorylation. Here, we report inositol pyrophosphates as novel regulators of cytoplasmic dynein-driven vesicle transport. Mammalian cells lacking IP6K1 display defects in dynein-dependent trafficking pathways, including endosomal sorting, vesicle movement, and Golgi maintenance. Expression of catalytically active but not inactive IP6K1 reverses these defects, suggesting a role for inositol pyrophosphates in these processes. Endosomes derived from slime mold lacking inositol pyrophosphates also display reduced dynein-directed microtubule transport. We demonstrate that Ser51 in the dynein intermediate chain (IC) is a target for pyrophosphorylation by IP7, and this modification promotes the interaction of the IC N-terminus with the p150(Glued) subunit of dynactin. IC-p150(Glued) interaction is decreased, and IC recruitment to membranes is reduced in cells lacking IP6K1. Our study provides the first evidence for the involvement of IP6Ks in dynein function and proposes that inositol pyrophosphate-mediated pyrophosphorylation may act as a regulatory signal to enhance dynein-driven transport.
Keywords: dynactin; dynein; inositol hexakisphosphate kinase 1; inositol pyrophosphates; protein pyrophosphorylation.
© 2016 The Author(s).
Figures



Comment in
-
Protein pyrophosphorylation: moving forward.Biochem J. 2016 Nov 1;473(21):3765-3768. doi: 10.1042/BCJ20160710C. Biochem J. 2016. PMID: 27789744 Free PMC article.
References
-
- Stephens L., Radenberg T., Thiel U., Vogel G., Khoo K.H., Dell A. et al. (1993) The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s). J. Biol. Chem. 268, 4009–4015 PMID: - PubMed
-
- Menniti F.S., Miller R.N., Putney J.W. Jr and Shears S.B. (1993) Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J. Biol. Chem. 268, 3850–3856 PMID: - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

