Loss of cargo binding in the human myosin VI deafness mutant (R1166X) leads to increased actin filament binding
- PMID: 27474411
- PMCID: PMC5074368
- DOI: 10.1042/BCJ20160571
Loss of cargo binding in the human myosin VI deafness mutant (R1166X) leads to increased actin filament binding
Abstract
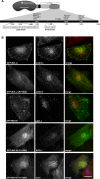
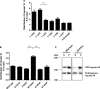
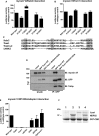
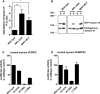
Mutations in myosin VI have been associated with autosomal-recessive (DFNB37) and autosomal-dominant (DFNA22) deafness in humans. Here, we characterise an myosin VI nonsense mutation (R1166X) that was identified in a family with hereditary hearing loss in Pakistan. This mutation leads to the deletion of the C-terminal 120 amino acids of the myosin VI cargo-binding domain, which includes the WWY-binding motif for the adaptor proteins LMTK2, Tom1 as well as Dab2. Interestingly, compromising myosin VI vesicle-binding ability by expressing myosin VI with the R1166X mutation or with single point mutations in the adaptor-binding sites leads to increased F-actin binding of this myosin in vitro and in vivo As our results highlight the importance of cargo attachment for regulating actin binding to the motor domain, we perform a detailed characterisation of adaptor protein binding and identify single amino acids within myosin VI required for binding to cargo adaptors. We not only show that the adaptor proteins can directly interact with the cargo-binding tail of myosin VI, but our in vitro studies also suggest that multiple adaptor proteins can bind simultaneously to non-overlapping sites in the myosin VI tail. In conclusion, our characterisation of the human myosin VI deafness mutant (R1166X) suggests that defects in cargo binding may leave myosin VI in a primed/activated state with an increased actin-binding ability.
Keywords: cargo binding; deafness; microfilaments; myosins.
© 2016 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

