The myeloid heat shock transcription factor 1/β-catenin axis regulates NLR family, pyrin domain-containing 3 inflammasome activation in mouse liver ischemia/reperfusion injury
- PMID: 27474884
- PMCID: PMC5074868
- DOI: 10.1002/hep.28739
The myeloid heat shock transcription factor 1/β-catenin axis regulates NLR family, pyrin domain-containing 3 inflammasome activation in mouse liver ischemia/reperfusion injury
Abstract
Heat shock transcription factor 1 (HSF1) has been implicated in the differential regulation of cell stress and disease states. β-catenin activation is essential for immune homeostasis. However, little is known about the role of macrophage HSF1-β-catenin signaling in the regulation of NLRP3 inflammasome activation during ischemia/reperfusion (I/R) injury (IRI) in the liver. This study investigated the functions and molecular mechanisms by which HSF1-β-catenin signaling influenced NLRP3-mediated innate immune response in vivo and in vitro. Using a mouse model of IR-induced liver inflammatory injury, we found that mice with a myeloid-specific HSF1 knockout (HSF1M-KO ) displayed exacerbated liver damage based on their increased serum alanine aminotransferase levels, intrahepatic macrophage/neutrophil trafficking, and proinflammatory interleukin (IL)-1β levels compared to the HSF1-proficient (HSF1FL/FL ) controls. Disruption of myeloid HSF1 markedly increased transcription factor X-box-binding protein (XBP1), NLR family, pyrin domain-containing 3 (NLRP3), and cleaved caspase-1 expression, which was accompanied by reduced β-catenin activity. Knockdown of XBP1 in HSF1-deficient livers using a XBP1 small interfering RNA ameliorated hepatocellular functions and reduced NLRP3/cleaved caspase-1 and IL-1β protein levels. In parallel in vitro studies, HSF1 overexpression increased β-catenin (Ser552) phosphorylation and decreased reactive oxygen species (ROS) production in bone-marrow-derived macrophages. However, myeloid HSF1 ablation inhibited β-catenin, but promoted XBP1. Furthermore, myeloid β-catenin deletion increased XBP1 messenger RNA splicing, whereas a CRISPR/CRISPR-associated protein 9-mediated XBP1 knockout diminished NLRP3/caspase-1.
Conclusion: The myeloid HSF1-β-catenin axis controlled NLRP3 activation by modulating the XBP1 signaling pathway. HSF1 activation promoted β-catenin, which, in turn, inhibited XBP1, leading to NLRP3 inactivation and reduced I/R-induced liver injury. These findings demonstrated that HSF1/β-catenin signaling is a novel regulator of innate immunity in liver inflammatory injury and implied the therapeutic potential for management of sterile liver inflammation in transplant recipients. (Hepatology 2016;64:1683-1698).
© 2016 by the American Association for the Study of Liver Diseases.
Figures


 ) and HSF1M-KO (
) and HSF1M-KO (
 ) mice. (A) Immunohistochemical staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4–6 mice/group. **p<0.01. (B) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and CXCL-10 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05. (C) Immunohistochemical staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4–6 mice/group. **p<0.01. (D) Quantitative RT-PCR-assisted detection of CXCL-1 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
) mice. (A) Immunohistochemical staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4–6 mice/group. **p<0.01. (B) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and CXCL-10 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05. (C) Immunohistochemical staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4–6 mice/group. **p<0.01. (D) Quantitative RT-PCR-assisted detection of CXCL-1 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
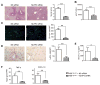
 ) or NLRP3 siRNA (
) or NLRP3 siRNA (
 ) (2 mg/kg) mixed with mannose-conjugated polymers at 4 h prior to ischemia. (A) Representative histological staining (H&E) of ischemic liver tissue. Results representative of 4–6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki’s histological grading. **p<0.01. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4–6 samples/group). **p<0.01. (C) Immunohistochemical staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4–6 mice/group. **p<0.01. (D) Immunohistochemical staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4–6 mice/group. **p<0.01. (E) ELISA analysis of IL-β levels in animal serum. Mean±SD (n=3–4 samples/group), *p<0.05. (F) Quantitative RT-PCR-assisted detection of mRNA coding for TNF-α and CXCL-10. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
) (2 mg/kg) mixed with mannose-conjugated polymers at 4 h prior to ischemia. (A) Representative histological staining (H&E) of ischemic liver tissue. Results representative of 4–6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki’s histological grading. **p<0.01. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4–6 samples/group). **p<0.01. (C) Immunohistochemical staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4–6 mice/group. **p<0.01. (D) Immunohistochemical staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4–6 mice/group. **p<0.01. (E) ELISA analysis of IL-β levels in animal serum. Mean±SD (n=3–4 samples/group), *p<0.05. (F) Quantitative RT-PCR-assisted detection of mRNA coding for TNF-α and CXCL-10. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
 ) or XBP1 siRNA (
) or XBP1 siRNA (
 ) (2 mg/kg) mixed with mannose-conjugated polymers at 4 h prior to ischemia. (A) Immunofluorescence staining of AlexaFluor488-labeled control siRNA (long arrow) and CD68 positive macrophages (short arrow) in ischemic liver lobes. Note: Green: AlexaFluor488-labeled siRNA; red: macrophage marker detected with CD68 mAb; blue: DAPI nuclear stain. Original magnification ×200; Representative of 3–4 mice/group (B) Representative histological staining (H&E) of ischemic liver tissue. Results representative of 4–6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki’s histological grading. **p<0.01. (C) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4–6 samples/group). **p<0.01. (D) ELISA analysis of IL-β levels in animal serum. Mean±SD (n=3–4 samples/group), *p<0.05. (E) Western blots analysis and relative density ratio of NLRP3 and cleaved caspase-1. Representative of three experiments. *p<0.05, **p<0.01. (F) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
) (2 mg/kg) mixed with mannose-conjugated polymers at 4 h prior to ischemia. (A) Immunofluorescence staining of AlexaFluor488-labeled control siRNA (long arrow) and CD68 positive macrophages (short arrow) in ischemic liver lobes. Note: Green: AlexaFluor488-labeled siRNA; red: macrophage marker detected with CD68 mAb; blue: DAPI nuclear stain. Original magnification ×200; Representative of 3–4 mice/group (B) Representative histological staining (H&E) of ischemic liver tissue. Results representative of 4–6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki’s histological grading. **p<0.01. (C) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4–6 samples/group). **p<0.01. (D) ELISA analysis of IL-β levels in animal serum. Mean±SD (n=3–4 samples/group), *p<0.05. (E) Western blots analysis and relative density ratio of NLRP3 and cleaved caspase-1. Representative of three experiments. *p<0.05, **p<0.01. (F) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents the mean±SD (n=3–4 samples/group). **p<0.01.
 ) or pBabe-HSF1 (
) or pBabe-HSF1 (
 ) followed by LPS (100ng/ml) stimulation. (A) Western blot analysis and relative density ratio of HSF1, p-Stat3 and p-β-catenin. Representative of three experiments. *p<0.05. (B) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents mean±SD (n=3–4 samples/group). **p<0.01. (C) ROS production was detected by Carboxy-H2DFFDA in LPS-stimulated BMMs from WT mice. Positive green fluorescent-labeled cells were counted blindly in 10 HPF/section (×200). Quantification of ROS-producing BMMs (green) per high power field (×200). **p<0.01. (D) BMMs from HSF1FL/FL (
) followed by LPS (100ng/ml) stimulation. (A) Western blot analysis and relative density ratio of HSF1, p-Stat3 and p-β-catenin. Representative of three experiments. *p<0.05. (B) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents mean±SD (n=3–4 samples/group). **p<0.01. (C) ROS production was detected by Carboxy-H2DFFDA in LPS-stimulated BMMs from WT mice. Positive green fluorescent-labeled cells were counted blindly in 10 HPF/section (×200). Quantification of ROS-producing BMMs (green) per high power field (×200). **p<0.01. (D) BMMs from HSF1FL/FL (
 ) and HSF1M-KO (
) and HSF1M-KO (
 ) mice were incubated with LPS (100 ng/ml). Western-assisted analysis and relative density ratio of HSF1, NLRP3, cleaved caspase-1, and p-β-catenin in LPS-stimulated cells. Representative of three experiments. *p<0.05. (E) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents mean±SD (n=3–4 samples/group). **p<0.01. (F) ROS production was detected by Carboxy-H2DFFDA in LPS-stimulated BMMs from HSF1FL/FL and HSF1M-KO mice. Positive green fluorescent-labeled cells were counted blindly in 10 HPF/section (×200). Quantification of ROS-producing BMMs (green) per high power field (×200). **p<0.01.
) mice were incubated with LPS (100 ng/ml). Western-assisted analysis and relative density ratio of HSF1, NLRP3, cleaved caspase-1, and p-β-catenin in LPS-stimulated cells. Representative of three experiments. *p<0.05. (E) Quantitative RT-PCR-assisted detection of mRNA coding for IL-1β, TNF-α, and CXCL-10. Each column represents mean±SD (n=3–4 samples/group). **p<0.01. (F) ROS production was detected by Carboxy-H2DFFDA in LPS-stimulated BMMs from HSF1FL/FL and HSF1M-KO mice. Positive green fluorescent-labeled cells were counted blindly in 10 HPF/section (×200). Quantification of ROS-producing BMMs (green) per high power field (×200). **p<0.01.
 ) and β-cateninM-KO (
) and β-cateninM-KO (
 ) mice were transfected with pBabe-HSF1 or control vector followed by LPS (100 ng/ml) stimulation. (A) Western-assisted analysis and relative density ratio of HSF1, β-catenin, and TLR4, NLRP3, and cleaved caspase-1. Representative of three experiments. *p<0.05, **p<0.01. (B) Western blot analysis and their relative density ratio of TRAF6, p-IRE1α, and XBP1s. Representative of three experiments. *p<0.05, **p<0.01. (C) ELISA-assisted production of IL-1β in cell culture supernatants. Mean±SD (n=3–4 samples/group). *p< 0.05. (D) BMMs from HSF1M-KO mice were transduced with lentivirus-expressing β-catenin (LV-pSin-β-catenin (
) mice were transfected with pBabe-HSF1 or control vector followed by LPS (100 ng/ml) stimulation. (A) Western-assisted analysis and relative density ratio of HSF1, β-catenin, and TLR4, NLRP3, and cleaved caspase-1. Representative of three experiments. *p<0.05, **p<0.01. (B) Western blot analysis and their relative density ratio of TRAF6, p-IRE1α, and XBP1s. Representative of three experiments. *p<0.05, **p<0.01. (C) ELISA-assisted production of IL-1β in cell culture supernatants. Mean±SD (n=3–4 samples/group). *p< 0.05. (D) BMMs from HSF1M-KO mice were transduced with lentivirus-expressing β-catenin (LV-pSin-β-catenin (
 ), LV-CRISPR/Cas9 XBP1 knockout (KO) (
), LV-CRISPR/Cas9 XBP1 knockout (KO) (
 ), or LV-pLJM1-GFP controls (
), or LV-pLJM1-GFP controls (
 ). After 24–48 h, cells were supplemented with 100 ng/ml of LPS for additional 6 h. Western blot analysis and their relative density ratio of β-catenin, p-IRE1α, XBP1s, NLRP3, and cleaved caspase-1 in LV-pSin-β-catenin- or LV-pLJM1-GFP-transduced cells. Representative of three experiments. **p<0.01. (E) Western blot analysis and their relative density ratio of XBP1s, NLRP3, and cleaved caspase-1 in LV-CRISPR/Cas9-XBP1 KO- or LV-pLJM1-GFP-transduced cells. Representative of three experiments. **p<0.01. (F) ELISA-assisted production of IL-β in cell culture supernatants. Mean±SD (n=3–4 samples/group). **p< 0.01.
). After 24–48 h, cells were supplemented with 100 ng/ml of LPS for additional 6 h. Western blot analysis and their relative density ratio of β-catenin, p-IRE1α, XBP1s, NLRP3, and cleaved caspase-1 in LV-pSin-β-catenin- or LV-pLJM1-GFP-transduced cells. Representative of three experiments. **p<0.01. (E) Western blot analysis and their relative density ratio of XBP1s, NLRP3, and cleaved caspase-1 in LV-CRISPR/Cas9-XBP1 KO- or LV-pLJM1-GFP-transduced cells. Representative of three experiments. **p<0.01. (F) ELISA-assisted production of IL-β in cell culture supernatants. Mean±SD (n=3–4 samples/group). **p< 0.01.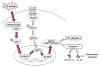
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

