Functional Effects of Cigarette Smoke-Induced Changes in Airway Smooth Muscle Mitochondrial Morphology
- PMID: 27474898
- PMCID: PMC5247322
- DOI: 10.1002/jcp.25508
Functional Effects of Cigarette Smoke-Induced Changes in Airway Smooth Muscle Mitochondrial Morphology
Abstract
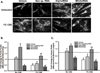
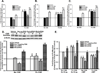
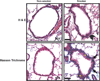
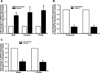
Long-term exposure to cigarette smoke (CS) triggers airway hyperreactivity and remodeling, effects that involve airway smooth muscle (ASM). We previously showed that CS destabilizes the networked morphology of mitochondria in human ASM by regulating the expression of mitochondrial fission and fusion proteins via multiple signaling mechanisms. Emerging data link regulation of mitochondrial morphology to cellular structure and function. We hypothesized that CS-induced changes in ASM mitochondrial morphology detrimentally affect mitochondrial function, leading to CS effects on contractility and remodeling. Here, ASM cells were exposed to 1% cigarette smoke extract (CSE) for 48 h to alter mitochondrial fission/fusion, or by inhibiting the fission protein Drp1 or the fusion protein Mfn2. Mitochondrial function was assessed via changes in bioenergetics or altered rates of proliferation and apoptosis. Our results indicate that both exposure to CS and inhibition of mitochondrial fission/fusion proteins affect mitochondrial function (i.e., energy metabolism, proliferation, and apoptosis) in ASM cells. In vivo, the airways in mice chronically exposed to CS are thickened and fibrotic, and the expression of proteins involved in mitochondrial function is dramatically altered in the ASM of these mice. We conclude that CS-induced changes in mitochondrial morphology (fission/fusion balance) correlate with mitochondrial function, and thus may control ASM proliferation, which plays a central role in airway health. J. Cell. Physiol. 232: 1053-1068, 2017. © 2016 Wiley Periodicals, Inc.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
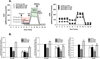
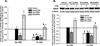

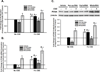

References
-
- Eisner MD. Environmental tobacco smoke and adult asthma. Exp Lung Res. 2005;31(Suppl 1):8–14. - PubMed
-
- Feinson JA, Chidekel AS. Adult smoking and environmental tobacco smoke: a persistent health threat to children. Del Med J. 2006;78:213–218. - PubMed
-
- Lodrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. - PubMed
-
- Omini C, Hernandez A, Zuccari G, Clavenna G, Daffonchio L. Passive cigarette smoke exposure induces airway hyperreactivity to histamine but not to acetylcholine in guinea-pigs. Pulm Pharmacol. 1990;3:145–150. - PubMed
-
- Thomson NC. The role of environmental tobacco smoke in the origins and progression of asthma. Curr Allergy Asthma Rep. 2007;7:303–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

