Sulfotyrosine dipeptide: Synthesis and evaluation as HIV-entry inhibitor
- PMID: 27475281
- PMCID: PMC5922769
- DOI: 10.1016/j.bioorg.2016.07.012
Sulfotyrosine dipeptide: Synthesis and evaluation as HIV-entry inhibitor
Abstract
Human immunodeficiency virus type 1 (HIV-1) is responsible for the worldwide AIDS pandemic. Due to the lack of prophylactic HIV-1 vaccine, drug treatment of the infected patients becomes essential to reduce the viral load and to slow down progression of the disease. Because of drug resistance, finding new antiviral agents is necessary for AIDS drug therapies. The interaction of gp120 and co-receptor (CCR5/CXCR4) mediates the entry of HIV-1 into host cells, which has been increasingly exploited in recent years as the target for new antiviral agents. A conserved co-receptor binding site on gp120 that recognizes sulfotyrosine (sTyr) residues represents a structural target to design novel HIV entry inhibitors. In this work, we developed an efficient synthesis of sulfotyrosine dipeptide and evaluated it as an HIV-1 entry inhibitor.
Keywords: Antiviral therapy; HIV entry inhibitor; Protein sulfation; Sulfopeptide; Sulfotyrosine; Sulfotyrosine dipeptide; gp120.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

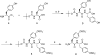
Similar articles
-
Design and synthesis of small molecule-sulfotyrosine mimetics that inhibit HIV-1 entry.Bioorg Med Chem. 2016 Apr 15;24(8):1718-28. doi: 10.1016/j.bmc.2016.02.044. Epub 2016 Mar 3. Bioorg Med Chem. 2016. PMID: 26968647 Free PMC article.
-
Dipeptide derivatives of AZT: synthesis, chemical stability, activation in human plasma, hPEPT1 affinity, and antiviral activity.ChemMedChem. 2008 Jun;3(6):970-8. doi: 10.1002/cmdc.200800012. ChemMedChem. 2008. PMID: 18389514
-
Design, Synthesis, and Antiviral activity of 1,2,3,4-Tetrahydropyrimidine derivatives acting as novel entry inhibitors to target at "Phe43 cavity" of HIV-1 gp120.Bioorg Med Chem. 2021 Dec 15;52:116526. doi: 10.1016/j.bmc.2021.116526. Epub 2021 Nov 20. Bioorg Med Chem. 2021. PMID: 34839157
-
Rational design of HIV-1 entry inhibitors.Methods Mol Biol. 2013;993:185-204. doi: 10.1007/978-1-62703-342-8_13. Methods Mol Biol. 2013. PMID: 23568472 Review.
-
HIV entry inhibitors: progress in development and application.Yao Xue Xue Bao. 2010 Feb;45(2):131-40. Yao Xue Xue Bao. 2010. PMID: 21348414 Review.
References
-
- UNAIDS. Data and analysis. 2014 DOI. http://www.unaids.org/en/
-
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. A Blueprint for HIV Vaccine Discovery. Cell host & microbe. 2012;12:396–407. - PMC - PubMed
-
- Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine. 2012;30:4310–4315. - PubMed
-
- Haqqani AA, Tilton JC. Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral research. 2013;98:158–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

