Communication: Contrasting effects of glycerol and DMSO on lipid membrane surface hydration dynamics and forces
- PMID: 27475340
- PMCID: PMC4967073
- DOI: 10.1063/1.4959904
Communication: Contrasting effects of glycerol and DMSO on lipid membrane surface hydration dynamics and forces
Abstract
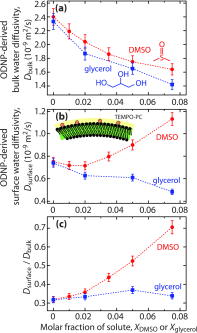
Glycerol and dimethyl sulfoxide (DMSO) are commonly used cryoprotectants in cellular systems, but due to the challenges of measuring the properties of surface-bound solvent, fundamental questions remain regarding the concentration, interactions, and conformation of these solutes at lipid membrane surfaces. We measured the surface water diffusivity at gel-phase dipalmitoylphosphatidylcholine (DPPC) bilayer surfaces in aqueous solutions containing ≤7.5 mol. % of DMSO or glycerol using Overhauser dynamic nuclear polarization. We found that glycerol similarly affects the diffusivity of water near the bilayer surface and that in the bulk solution (within 20%), while DMSO substantially increases the diffusivity of surface water relative to bulk water. We compare these measurements of water dynamics with those of equilibrium forces between DPPC bilayers in the same solvent mixtures. DMSO greatly decreases the range and magnitude of the repulsive forces between the bilayers, whereas glycerol increases it. We propose that the differences in hydrogen bonding capability of the two solutes leads DMSO to dehydrate the lipid head groups, while glycerol affects surface hydration only as much as it affects the bulk water properties. The results suggest that the mechanism of the two most common cryoprotectants must be fundamentally different: in the case of DMSO by decoupling the solvent from the lipid surface, and in the case of glycerol by altering the hydrogen bond structure and intermolecular cohesion of the global solvent, as manifested by increased solvent viscosity.
Figures

Similar articles
-
Correlating steric hydration forces with water dynamics through surface force and diffusion NMR measurements in a lipid-DMSO-H2O system.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):10708-13. doi: 10.1073/pnas.1512325112. Epub 2015 Aug 10. Proc Natl Acad Sci U S A. 2015. PMID: 26261313 Free PMC article.
-
Molecular dynamics simulation of DPPC bilayer in DMSO.Biophys J. 1999 May;76(5):2472-8. doi: 10.1016/S0006-3495(99)77402-3. Biophys J. 1999. PMID: 10233064 Free PMC article.
-
Lipid membrane structure and interactions in dimethyl sulfoxide/water mixtures.Biophys J. 1998 Nov;75(5):2343-51. doi: 10.1016/S0006-3495(98)77678-7. Biophys J. 1998. PMID: 9788929 Free PMC article.
-
Effects of Dimethyl Sulfoxide on Surface Water near Phospholipid Bilayers.Biophys J. 2016 Dec 6;111(11):2481-2491. doi: 10.1016/j.bpj.2016.10.033. Biophys J. 2016. PMID: 27926849 Free PMC article.
-
DMSO induces dehydration near lipid membrane surfaces.Biophys J. 2015 Jul 21;109(2):330-9. doi: 10.1016/j.bpj.2015.06.011. Biophys J. 2015. PMID: 26200868 Free PMC article.
Cited by
-
AFM nanoindentation reveals decrease of elastic modulus of lipid bilayers near freezing point of water.Sci Rep. 2019 Dec 19;9(1):19473. doi: 10.1038/s41598-019-55519-7. Sci Rep. 2019. PMID: 31857622 Free PMC article.
-
Dynamic Nuclear Polarization of Biomembrane Assemblies.Biomolecules. 2020 Aug 27;10(9):1246. doi: 10.3390/biom10091246. Biomolecules. 2020. PMID: 32867275 Free PMC article.
-
A biradical-tagged phospholipid as a polarizing agent for solid-state MAS Dynamic Nuclear Polarization NMR of membrane proteins.Solid State Nucl Magn Reson. 2019 Aug;100:92-101. doi: 10.1016/j.ssnmr.2019.04.003. Epub 2019 Apr 17. Solid State Nucl Magn Reson. 2019. PMID: 31029957 Free PMC article.
-
The Role of Cryoprotective Agents in Liposome Stabilization and Preservation.Int J Mol Sci. 2022 Oct 18;23(20):12487. doi: 10.3390/ijms232012487. Int J Mol Sci. 2022. PMID: 36293340 Free PMC article. Review.
-
In-Cell NMR of Intact Mammalian Cells Preserved with the Cryoprotectants DMSO and Glycerol Have Similar DNP Performance.Front Mol Biosci. 2022 Jan 25;8:789478. doi: 10.3389/fmolb.2021.789478. eCollection 2021. Front Mol Biosci. 2022. PMID: 35145995 Free PMC article.
References
-
- Surewicz W. K., Chem. Phys. Lipids 34, 363 (1984).10.1016/0009-3084(84)90010-0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

