A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection
- PMID: 27476412
- PMCID: PMC4993926
- DOI: 10.1016/j.chom.2016.07.004
A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection
Abstract
Currently there are no approved vaccines or specific therapies to prevent or treat Zika virus (ZIKV) infection. We interrogated a library of FDA-approved drugs for their ability to block infection of human HuH-7 cells by a newly isolated ZIKV strain (ZIKV MEX_I_7). More than 20 out of 774 tested compounds decreased ZIKV infection in our in vitro screening assay. Selected compounds were further validated for inhibition of ZIKV infection in human cervical, placental, and neural stem cell lines, as well as primary human amnion cells. Established anti-flaviviral drugs (e.g., bortezomib and mycophenolic acid) and others that had no previously known antiviral activity (e.g., daptomycin) were identified as inhibitors of ZIKV infection. Several drugs reduced ZIKV infection across multiple cell types. This study identifies drugs that could be tested in clinical studies of ZIKV infection and provides a resource of small molecules to study ZIKV pathogenesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
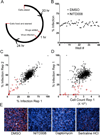

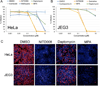

Comment in
-
Zika and Other Emerging Viruses: Aiming at the Right Target.Cell Host Microbe. 2016 Oct 12;20(4):420-422. doi: 10.1016/j.chom.2016.09.011. Cell Host Microbe. 2016. PMID: 27736642 Free PMC article.
Similar articles
-
Identification of anti-flaviviral drugs with mosquitocidal and anti-Zika virus activity in Aedes aegypti.PLoS Negl Trop Dis. 2019 Aug 20;13(8):e0007681. doi: 10.1371/journal.pntd.0007681. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31430351 Free PMC article.
-
Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay.Antiviral Res. 2017 Feb;138:47-56. doi: 10.1016/j.antiviral.2016.11.018. Epub 2016 Dec 3. Antiviral Res. 2017. PMID: 27919709
-
Identification of Inhibitors of ZIKV Replication.Viruses. 2020 Sep 18;12(9):1041. doi: 10.3390/v12091041. Viruses. 2020. PMID: 32961956 Free PMC article.
-
Strategies for Zika drug discovery.Curr Opin Virol. 2019 Apr;35:19-26. doi: 10.1016/j.coviro.2019.01.005. Epub 2019 Mar 7. Curr Opin Virol. 2019. PMID: 30852345 Review.
-
Investigational drugs for the treatment of Zika virus infection: a preclinical and clinical update.Expert Opin Investig Drugs. 2018 Dec;27(12):951-962. doi: 10.1080/13543784.2018.1548609. Expert Opin Investig Drugs. 2018. PMID: 30430882 Review.
Cited by
-
Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats.Sci Rep. 2020 Oct 13;10(1):17073. doi: 10.1038/s41598-020-74084-y. Sci Rep. 2020. PMID: 33051517 Free PMC article.
-
Crosstalk between nucleocytoplasmic trafficking and the innate immune response to viral infection.J Biol Chem. 2021 Jul;297(1):100856. doi: 10.1016/j.jbc.2021.100856. Epub 2021 Jun 29. J Biol Chem. 2021. PMID: 34097873 Free PMC article. Review.
-
Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies.Biomedicines. 2023 Dec 15;11(12):3316. doi: 10.3390/biomedicines11123316. Biomedicines. 2023. PMID: 38137537 Free PMC article. Review.
-
A Systems Biology Workflow for Drug and Vaccine Repurposing: Identifying Small-Molecule BCG Mimics to Reduce or Prevent COVID-19 Mortality.Pharm Res. 2020 Oct 6;37(11):212. doi: 10.1007/s11095-020-02930-9. Pharm Res. 2020. PMID: 33025261 Free PMC article.
-
Identification of anti-flaviviral drugs with mosquitocidal and anti-Zika virus activity in Aedes aegypti.PLoS Negl Trop Dis. 2019 Aug 20;13(8):e0007681. doi: 10.1371/journal.pntd.0007681. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31430351 Free PMC article.
References
-
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. - PubMed
-
- Baltz RH. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Current opinion in chemical biology. 2009;13:144–151. - PubMed
-
- Benelli G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitology research. 2015;114:3201–3212. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

