Gut Microbial Metabolites Fuel Host Antibody Responses
- PMID: 27476413
- PMCID: PMC4982788
- DOI: 10.1016/j.chom.2016.07.001
Gut Microbial Metabolites Fuel Host Antibody Responses
Abstract
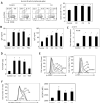
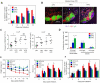
Antibody production is a metabolically demanding process that is regulated by gut microbiota, but the microbial products supporting B cell responses remain incompletely identified. We report that short-chain fatty acids (SCFAs), produced by gut microbiota as fermentation products of dietary fiber, support host antibody responses. In B cells, SCFAs increase acetyl-CoA and regulate metabolic sensors to increase oxidative phosphorylation, glycolysis, and fatty acid synthesis, which produce energy and building blocks supporting antibody production. In parallel, SCFAs control gene expression to express molecules necessary for plasma B cell differentiation. Mice with low SCFA production due to reduced dietary fiber consumption or microbial insufficiency are defective in homeostatic and pathogen-specific antibody responses, resulting in greater pathogen susceptibility. However, SCFA or dietary fiber intake restores this immune deficiency. This B cell-helping function of SCFAs is detected from the intestines to systemic tissues and conserved among mouse and human B cells, highlighting its importance.
Keywords: B cells; antibody; metabolism; short-chain fatty acids.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

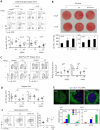
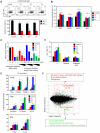
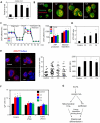
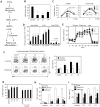
Comment in
-
B cell-helping functions of gut microbial metabolites.Microb Cell. 2016 Sep 23;3(10):529-531. doi: 10.15698/mic2016.10.536. Microb Cell. 2016. PMID: 28357321 Free PMC article.
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature reviews Immunology. 2010;10:131–144. - PubMed
-
- Aldrich MB, Chen W, Blackburn MR, Martinez-Valdez H, Datta SK, Kellems RE. Impaired germinal center maturation in adenosine deaminase deficiency. Journal of immunology. 2003;171:5562–5570. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

