Innervation of taste buds revealed with Brainbow-labeling in mouse
- PMID: 27476649
- PMCID: PMC5108162
- DOI: 10.1111/joa.12527
Innervation of taste buds revealed with Brainbow-labeling in mouse
Abstract
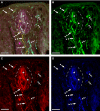
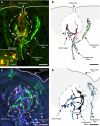
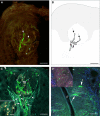
Nerve fibers that surround and innervate the taste bud were visualized with inherent fluorescence using Brainbow transgenic mice that were generated by mating the founder line L with nestin-cre mice. Multicolor fluorescence revealed perigemmal fibers as branched within the non-taste epithelium and ending in clusters of multiple rounded swellings surrounding the taste pore. Brainbow-labeling also revealed the morphology and branching pattern of single intragemmal fibers. These taste bud fibers frequently innervated both the peripheral bud, where immature gemmal cells are located, and the central bud, where mature, differentiated cells are located. The fibers typically bore preterminal and terminal swellings, growth cones with filopodia, swellings, and rounded retraction bulbs. These results establish an anatomical substrate for taste nerve fibers to contact and remodel among receptor cells at all stages of their differentiation, an interpretation that was supported by staining with GAP-43, a marker for growing fibers and growth cones.
Keywords: geniculate ganglion; innervation; morphology; mouse; plasticity; taste bud.
© 2016 Anatomical Society.
Figures





References
-
- Benowitz LI, Routtenberg A (1997) GAP‐43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20, 84–91. - PubMed
-
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G (1999) Localization of ATP‐gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport 10, 1107–1111. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

