NLRP3 protects alveolar barrier integrity by an inflammasome-independent increase of epithelial cell adherence
- PMID: 27476670
- PMCID: PMC4967923
- DOI: 10.1038/srep30943
NLRP3 protects alveolar barrier integrity by an inflammasome-independent increase of epithelial cell adherence
Abstract
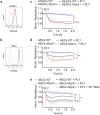
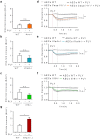
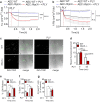
Bacterial pneumonia is a major cause of acute lung injury and acute respiratory distress syndrome, characterized by alveolar barrier disruption. NLRP3 is best known for its ability to form inflammasomes and to regulate IL-1β and IL-18 production in myeloid cells. Here we show that NLRP3 protects the integrity of the alveolar barrier in a mouse model of Streptococcus pneumoniae-induced pneumonia, and ex vivo upon treatment of isolated perfused and ventilated lungs with the purified bacterial toxin, pneumolysin. We reveal that the preserving effect of NLRP3 on the lung barrier is independent of inflammasomes, IL-1β and IL-18. NLRP3 improves the integrity of alveolar epithelial cell monolayers by enhancing cellular adherence. Collectively, our study uncovers a novel function of NLRP3 by demonstrating that it protects epithelial barrier function independently of inflammasomes.
Figures

Similar articles
-
The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia.J Immunol. 2011 Jul 1;187(1):434-40. doi: 10.4049/jimmunol.1003143. Epub 2011 Jun 6. J Immunol. 2011. PMID: 21646297
-
NLRP3 and ASC differentially affect the lung transcriptome during pneumococcal pneumonia.Am J Respir Cell Mol Biol. 2014 Apr;50(4):699-712. doi: 10.1165/rcmb.2013-0015OC. Am J Respir Cell Mol Biol. 2014. PMID: 24164497
-
Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux.J Immunol. 2015 Feb 15;194(4):1763-75. doi: 10.4049/jimmunol.1401624. Epub 2015 Jan 21. J Immunol. 2015. PMID: 25609842 Free PMC article.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
-
Implications of Inflammasomes in Human Diseases: NLRP3 Inflammasome and Animal Models.Cornea. 2018 Nov;37 Suppl 1:S86-S90. doi: 10.1097/ICO.0000000000001717. Cornea. 2018. PMID: 30211747 Review.
Cited by
-
Novel Role for Tranilast in Regulating NLRP3 Ubiquitination, Vascular Inflammation, and Atherosclerosis.J Am Heart Assoc. 2020 Jun 16;9(12):e015513. doi: 10.1161/JAHA.119.015513. Epub 2020 Jun 1. J Am Heart Assoc. 2020. PMID: 32476536 Free PMC article.
-
Soluble mucus component CLCA1 modulates expression of leukotactic cytokines and BPIFA1 in murine alveolar macrophages but not in bone marrow-derived macrophages.Histochem Cell Biol. 2018 Jun;149(6):619-633. doi: 10.1007/s00418-018-1664-y. Epub 2018 Apr 2. Histochem Cell Biol. 2018. PMID: 29610986 Free PMC article.
-
Cytokine-Ion Channel Interactions in Pulmonary Inflammation.Front Immunol. 2018 Jan 4;8:1644. doi: 10.3389/fimmu.2017.01644. eCollection 2017. Front Immunol. 2018. PMID: 29354115 Free PMC article. Review.
-
NLRP3 Inflammasome Mediates Dormant Neutrophil Recruitment following Sterile Lung Injury and Protects against Subsequent Bacterial Pneumonia in Mice.Front Immunol. 2017 Oct 31;8:1337. doi: 10.3389/fimmu.2017.01337. eCollection 2017. Front Immunol. 2017. PMID: 29163464 Free PMC article.
-
The role of respiratory epithelium in host defence against influenza virus infection.Biomed J. 2018 Aug;41(4):218-233. doi: 10.1016/j.bj.2018.08.004. Epub 2018 Sep 10. Biomed J. 2018. PMID: 30348265 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

