Induction Chemotherapy is Not Superior to a Surgery-First Strategy for Clinical N1 Non-Small Cell Lung Cancer
- PMID: 27476819
- PMCID: PMC5140084
- DOI: 10.1016/j.athoracsur.2016.05.065
Induction Chemotherapy is Not Superior to a Surgery-First Strategy for Clinical N1 Non-Small Cell Lung Cancer
Abstract
Background: Guidelines recommend primary surgical resection for non-small cell lung cancer (NSCLC) patients with clinical N1 disease and adjuvant chemotherapy if nodal disease is confirmed after resection. We tested the hypothesis that induction chemotherapy for clinical N1 (cN1) disease improves survival.
Methods: Patients treated with lobectomy or pneumonectomy for cT1-3 N1 M0 NSCLC from 2006 to 2011 in the National Cancer Data Base were stratified by treatment strategy: surgery first vs induction chemotherapy. Propensity scores were developed and matched with a 2:1 nearest neighbor algorithm. Survival analyses using Kaplan-Meier methods were performed on the unadjusted and propensity-matched cohorts.
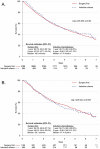
Results: A total of 5,364 cN1 patients were identified for inclusion, of which 565 (10.5%) were treated with induction chemotherapy. Clinical nodal staging was accurate in 68.6% (n = 3,292) of patients treated with surgical resection first, whereas 16.3% (n = 780) were pN0 and 10.7% (n = 514) were pN2-3. Adjuvant chemotherapy was given to 60.9% of the surgery-first patients who were pN1-3 after resection. Before adjustment, patients treated with induction chemotherapy were younger, with lower comorbidity burden, were more likely to be treated at an academic center and to have private insurance (all p < 0.001), but were significantly more likely to have T3 tumors (28.7% vs 9.9%, p < 0.001) and to require pneumonectomy (23.5% vs 18.5%, p = 0.005). The unadjusted and propensity-matched analyses found no differences in short-term outcomes or survival between groups.
Conclusions: Induction chemotherapy for cN1 NSCLC is not associated with improved survival. This finding supports the currently recommended treatment paradigm of surgery first for cN1 NSCLC.
Copyright © 2016 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis.J Thorac Cardiovasc Surg. 2016 May;151(5):1380-8. doi: 10.1016/j.jtcvs.2015.12.028. Epub 2015 Dec 21. J Thorac Cardiovasc Surg. 2016. PMID: 26874598 Free PMC article.
-
Problems in diagnosis and surgical management of clinical N1 non-small cell lung cancer.Ann Thorac Surg. 2005 May;79(5):1682-5. doi: 10.1016/j.athoracsur.2004.11.025. Ann Thorac Surg. 2005. PMID: 15854954
-
Induction chemotherapy for T3N0M0 non-small-cell lung cancer increases the rate of complete resection but does not confer improved survival.Eur J Cardiothorac Surg. 2017 Aug 1;52(2):370-377. doi: 10.1093/ejcts/ezx091. Eur J Cardiothorac Surg. 2017. PMID: 28402406 Free PMC article.
-
The present status of surgery for lung cancer.Acta Chir Belg. 1996 Nov-Dec;96(6):245-51. Acta Chir Belg. 1996. PMID: 9008764 Review.
-
Should aggressive surgery ever be part of the management of small cell lung cancer?Thorac Surg Clin. 2004 May;14(2):271-81. doi: 10.1016/S1547-4127(04)00004-0. Thorac Surg Clin. 2004. PMID: 15382303 Review.
Cited by
-
The Role of Adjuvant Chemotherapy in pN1 (IIB/IIIA) NSCLC Patients Who Undergo Pneumonectomy: Is It Still Justified in the Modern Era?Cancers (Basel). 2024 Aug 31;16(17):3041. doi: 10.3390/cancers16173041. Cancers (Basel). 2024. PMID: 39272899 Free PMC article.
-
Preoperative identification of clinicopathological prognostic factors for relapse-free survival in clinical N1 non-small cell lung cancer: a retrospective single center-based study.J Cardiothorac Surg. 2020 Aug 28;15(1):229. doi: 10.1186/s13019-020-01272-2. J Cardiothorac Surg. 2020. PMID: 32859238 Free PMC article.
-
Approach to Resectable N1 Non-Small Cell Lung Cancer: An Analysis of the National Cancer Database.J Surg Res. 2021 Mar;259:145-153. doi: 10.1016/j.jss.2020.11.024. Epub 2020 Dec 3. J Surg Res. 2021. PMID: 33279840 Free PMC article.
-
Primary pneumonectomy, pneumonectomy after induction therapy, and salvage pneumonectomy: a comparison of surgical and prognostic outcomes.J Thorac Dis. 2020 May;12(5):2672-2682. doi: 10.21037/jtd.2020.03.19. J Thorac Dis. 2020. PMID: 32642175 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. - PubMed
-
- National Comprehensive Cancer Network [Accessed January 18, 2016];Non-Small Cell Lung Cancer (Version 4.2016) Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
-
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. - PubMed
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

