Assessing protocol adherence in a clinical trial with ordered treatment regimens: Quantifying the pragmatic, randomized optimal platelet and plasma ratios (PROPPR) trial experience
- PMID: 27476886
- PMCID: PMC5050112
- DOI: 10.1016/j.injury.2016.07.028
Assessing protocol adherence in a clinical trial with ordered treatment regimens: Quantifying the pragmatic, randomized optimal platelet and plasma ratios (PROPPR) trial experience
Abstract
Background: Medication dispensing errors are common in clinical trials, and have a significant impact on the quality and validity of a trial. Therefore, the definition, calculation and evaluation of such errors are important for supporting a trial's conclusions. A variety of medication dispensing errors can occur. In this paper, we focus on errors in trials where the intervention includes multiple therapies that must be given in a pre-specified order that varies across treatment arms and varies in duration.
Methods: The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial was a Phase III multi-site, randomized trial to compare the effectiveness and safety of 1:1:1 transfusion ratios of plasma and platelets to red blood cells with a 1:1:2 ratio. In this trial, these three types of blood products were to be transfused in a pre-defined order that differed by treatment arm. In this paper, we present approaches from the PROPPR trial that we used to define and calculate the occurrence of out of order blood transfusion errors. We applied the proposed method to calculate protocol adherence to the specified order of transfusion in each treatment arm.
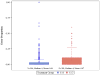
Results: Using our proposed method, protocol adherence was greater in the 1:1:1 group than in the 1:1:2 group (96% vs 93%) (p<0.0001), although out of order transfusion errors in both groups were low. Final transfusion ratios of plasma to platelets to red blood cells for the 1:1:1 ratio group was 0.93:1.32:1, while the transfusion ratio for the 1:1:2 ratio group was 0.48:0.48:1.
Conclusions: Overall, PROPPR adherence to blood transfusion order pre-specified in the protocol was high, and the required order of transfusions for the 1:1:2 group was more difficult to achieve. The approaches proposed in this manuscript were useful in evaluating the PROPPR adherence and are potentially useful for other trials where a specific treatment orders with varying durations must be maintained.
Keywords: Blood product transfusion; Medication dispensing error; Ordered treatment regimens; Protocol adherence.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Statement The authors have no declared conflicts of interest related to this manuscript.
Figures


Similar articles
-
The pragmatic randomized optimal platelet and plasma ratios trial: what does it mean for remote damage control resuscitation?Transfusion. 2016 Apr;56 Suppl 2:S149-56. doi: 10.1111/trf.13502. Transfusion. 2016. PMID: 27100751
-
Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial.JAMA. 2015 Feb 3;313(5):471-82. doi: 10.1001/jama.2015.12. JAMA. 2015. PMID: 25647203 Free PMC article. Clinical Trial.
-
Dynamic impact of transfusion ratios on outcomes in severely injured patients: Targeted machine learning analysis of the Pragmatic, Randomized Optimal Platelet and Plasma Ratios randomized clinical trial.J Trauma Acute Care Surg. 2020 Sep;89(3):505-513. doi: 10.1097/TA.0000000000002819. J Trauma Acute Care Surg. 2020. PMID: 32520897 Free PMC article. Clinical Trial.
-
Massive transfusion protocol in adult trauma population.Am J Emerg Med. 2020 Dec;38(12):2661-2666. doi: 10.1016/j.ajem.2020.07.041. Epub 2020 Jul 22. Am J Emerg Med. 2020. PMID: 33071074 Review.
-
Massive transfusion: red blood cell to plasma and platelet unit ratios for resuscitation of massive hemorrhage.Curr Opin Hematol. 2015 Nov;22(6):533-9. doi: 10.1097/MOH.0000000000000184. Curr Opin Hematol. 2015. PMID: 26390160 Review.
Cited by
-
Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial.Blood Adv. 2018 Jul 24;2(14):1696-1704. doi: 10.1182/bloodadvances.2018017699. Blood Adv. 2018. PMID: 30030268 Free PMC article. Clinical Trial.
-
Outcomes after concomitant traumatic brain injury and hemorrhagic shock: A secondary analysis from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios trial.J Trauma Acute Care Surg. 2017 Oct;83(4):668-674. doi: 10.1097/TA.0000000000001584. Epub 2017 Jun 6. J Trauma Acute Care Surg. 2017. PMID: 28930959 Free PMC article. Clinical Trial.
-
Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality.J Trauma Acute Care Surg. 2017 Jul;83(1):19-24. doi: 10.1097/TA.0000000000001531. J Trauma Acute Care Surg. 2017. PMID: 28452870 Free PMC article. Clinical Trial.
References
-
- Alexander JH, et al. Documentation of study medication dispensing in a prospective large randomized clinical trial: experiences from the ARISTOTLE Trial. American heart journal. 2013;166(3):559–565.e1. - PubMed
-
- James KL, et al. Incidence, type and causes of dispensing errors: a review of the literature. International journal of pharmacy practice. 2009;17(1):9–30. - PubMed
-
- SPENCER MG, SMITH AP. A multicentre study of dispensing errors in British hospitals. International Journal of Pharmacy Practice. 1993;2(3):142–146.
-
- Allan E, Barker K. Fundamentals of medication error research. American Journal of Health-System Pharmacy. 1990;47(3):555–571. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

