Cell-Free Mixing of Escherichia coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis
- PMID: 27476989
- PMCID: PMC6728267
- DOI: 10.1021/acssynbio.6b00154
Cell-Free Mixing of Escherichia coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis
Abstract
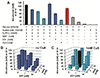
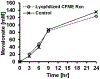
Cell-free metabolic engineering (CFME) is advancing a powerful paradigm for accelerating the design and synthesis of biosynthetic pathways. However, as most cell-free biomolecule synthesis systems to date use purified enzymes, energy and cofactor balance can be limiting. To address this challenge, we report a new CFME framework for building biosynthetic pathways by mixing multiple crude lysates, or extracts. In our modular approach, cell-free lysates, each selectively enriched with an overexpressed enzyme, are generated in parallel and then combinatorically mixed to construct a full biosynthetic pathway. Endogenous enzymes in the cell-free extract fuel high-level energy and cofactor regeneration. As a model, we apply our framework to synthesize mevalonate, an intermediate in isoprenoid synthesis. We use our approach to rapidly screen enzyme variants, optimize enzyme ratios, and explore cofactor landscapes for improving pathway performance. Further, we show that genomic deletions in the source strain redirect metabolic flux in resultant lysates. In an optimized system, mevalonate was synthesized at 17.6 g·L-1 (119 mM) over 20 h, resulting in a volumetric productivity of 0.88 g·L-1·hr-1. We also demonstrate that this system can be lyophilized and retain biosynthesis capability. Our system catalyzes ∼1250 turnover events for the cofactor NAD+ and demonstrates the ability to rapidly prototype and debug enzymatic pathways in vitro for compelling metabolic engineering and synthetic biology applications.
Keywords: Escherichia coli; cell-free metabolic engineering; cell-free synthetic biology; in vitro; metabolic pathway debugging; mevalonate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

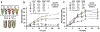


References
-
- Bohlmann J, and Keeling CI (2008) Terpenoid biomaterials, The Plant Journal 54, 656–669. - PubMed
-
- Leavell MD, McPhee DJ, and Paddon CJ (2016) Developing fermentative terpenoid production for commercial usage, Current opinion in biotechnology 37, 114–119. - PubMed
-
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Fleck M, Bajad S, Dang G, Dengrove D, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T, Jiang H, Kizer L, Lieu B, Melis D, Moss N, Regentin R, Secrest S, Tsuruta H, Vazquez R, Westblade LF, Xu L, Yu M, Zhang Y, Zhao L, Lievense J, Covello PS, Keasling JD, Reiling KK, Renninger NS, and Newman JD (2013) High-level semi-synthetic production of the potent antimalarial artemisinin, Nature 496, 528–532. - PubMed
-
- Zurbriggen A, Kirst H, and Melis A (2012) Isoprene production via the mevalonic acid pathway in Escherichia coli (Bacteria), BioEnergy Research 5, 814–828.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

