Analysis of Neuronal Spike Trains, Deconstructed
- PMID: 27477016
- PMCID: PMC4970242
- DOI: 10.1016/j.neuron.2016.05.039
Analysis of Neuronal Spike Trains, Deconstructed
Abstract
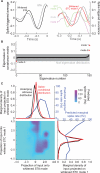
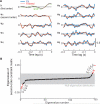
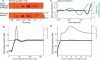
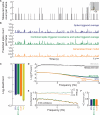
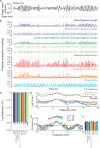
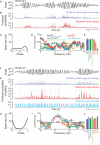
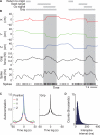
As information flows through the brain, neuronal firing progresses from encoding the world as sensed by the animal to driving the motor output of subsequent behavior. One of the more tractable goals of quantitative neuroscience is to develop predictive models that relate the sensory or motor streams with neuronal firing. Here we review and contrast analytical tools used to accomplish this task. We focus on classes of models in which the external variable is compared with one or more feature vectors to extract a low-dimensional representation, the history of spiking and other variables are potentially incorporated, and these factors are nonlinearly transformed to predict the occurrences of spikes. We illustrate these techniques in application to datasets of different degrees of complexity. In particular, we address the fitting of models in the presence of strong correlations in the external variable, as occurs in natural sensory stimuli and in movement. Spectral correlation between predicted and measured spike trains is introduced to contrast the relative success of different methods.
Keywords: dimensional reduction; feature vector; neuroinformatics; predictive modeling; reverse correlation; systems identification; time series.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




References
-
- Adelman TL, Bialek W, Olberg RM. The information content of receptive fields. Neuron. 2003;40:823–833. - PubMed
-
- Agüera y Arcas B, Fairhall AL. What causes a neuron to spike? Neural Comput. 2003;15:1789–1807. - PubMed
-
- Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Caski F, editors. Proceeding of the Second International Symposium on Information Theory. Akademiai Kiado; 1973. pp. 267–281.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

