Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging
- PMID: 27477268
- PMCID: PMC5034299
- DOI: 10.1016/j.bpj.2016.04.054
Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging
Abstract
Optical Ca(2+) indicators are powerful tools for investigating intracellular Ca(2+) signals in living cells. Although a variety of Ca(2+) indicators have been developed, deciphering the physiological functions and spatiotemporal dynamics of Ca(2+) in intracellular organelles remains challenging. Genetically encoded Ca(2+) indicators (GECIs) using fluorescent proteins are promising tools for organellar Ca(2+) imaging, and much effort has been devoted to their development. In this review, we first discuss the key points of organellar Ca(2+) imaging and summarize the requirements for optimal organellar Ca(2+) indicators. Then, we highlight some of the recent advances in the engineering of fluorescent GECIs targeted to specific organelles. Finally, we discuss the limitations of currently available GECIs and the requirements for advancing the research on intraorganellar Ca(2+) signaling.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
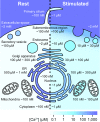

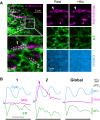

Comment in
-
New Biological Frontiers Illuminated by Molecular Sensors and Actuators.Biophys J. 2016 Sep 20;111(6):E01-E02. doi: 10.1016/j.bpj.2016.08.012. Biophys J. 2016. PMID: 27653492 Free PMC article. No abstract available.
References
-
- Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. - PubMed
-
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. - PubMed
-
- Rizzuto R., De Stefani D., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. - PubMed
-
- Takahashi A., Camacho P., Herman B. Measurement of intracellular calcium. Physiol. Rev. 1999;79:1089–1125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

