Immune dysregulation in patients with PTEN hamartoma tumor syndrome: Analysis of FOXP3 regulatory T cells
- PMID: 27477328
- PMCID: PMC5292998
- DOI: 10.1016/j.jaci.2016.03.059
Immune dysregulation in patients with PTEN hamartoma tumor syndrome: Analysis of FOXP3 regulatory T cells
Abstract
Background: Patients with heterozygous germline mutations in phosphatase and tensin homolog deleted on chromosome 10 (PTEN) experience autoimmunity and lymphoid hyperplasia.
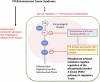
Objectives: Because regulation of the phosphoinositide 3-kinase (PI3K) pathway is critical for maintaining regulatory T (Treg) cell functions, we investigate Treg cells in patients with heterozygous germline PTEN mutations (PTEN hamartoma tumor syndrome [PHTS]).
Methods: Patients with PHTS were assessed for immunologic conditions, lymphocyte subsets, forkhead box P3 (FOXP3)+ Treg cell levels, and phenotype. To determine the functional importance of phosphatases that control the PI3K pathway, we assessed Treg cell induction in vitro, mitochondrial depolarization, and recruitment of PTEN to the immunologic synapse.
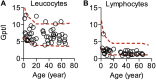
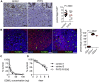
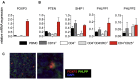
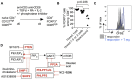
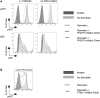
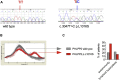
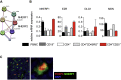
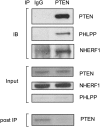
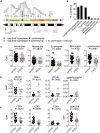
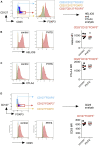
Results: Autoimmunity and peripheral lymphoid hyperplasia were found in 43% of 79 patients with PHTS. Immune dysregulation in patients with PHTS included lymphopenia, CD4+ T-cell reduction, and changes in T- and B-cell subsets. Although total CD4+FOXP3+ Treg cell numbers are reduced, frequencies are maintained in the blood and intestine. Despite pathogenic PTEN mutations, the FOXP3+ T cells are phenotypically normal. We show that the phosphatase PH domain leucine-rich repeat protein phosphatase (PHLPP) downstream of PTEN is highly expressed in normal human Treg cells and provides complementary phosphatase activity. PHLPP is indispensable for the differentiation of induced Treg cells in vitro and Treg cell mitochondrial fitness. PTEN and PHLPP form a phosphatase network that is polarized at the immunologic synapse.
Conclusion: Heterozygous loss of function of PTEN in human subjects has a significant effect on T- and B-cell immunity. Assembly of the PTEN-PHLPP phosphatase network allows coordinated phosphatase activities at the site of T-cell receptor activation, which is important for limiting PI3K hyperactivation in Treg cells despite PTEN haploinsufficiency.
Keywords: PH domain leucine-rich repeat protein phosphatase; PHTS; PTEN; Regulatory T cells; autoimmunity; immunologic synapse; phosphatases; phosphoinositide 3-kinase.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Suzuki A., Yamaguchi M.T., Ohteki T., Sasaki T., Kaisho T., Kimura Y. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

